Spatial origin of the extracellular ATP-induced cytosolic calcium signature in Arabidopsis thaliana roots: wave formation and variation with phosphate nutrition
- PMID: 35395136
- PMCID: PMC10286661
- DOI: 10.1111/plb.13427
Spatial origin of the extracellular ATP-induced cytosolic calcium signature in Arabidopsis thaliana roots: wave formation and variation with phosphate nutrition
Abstract
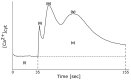
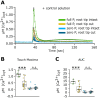
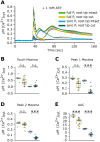
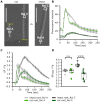
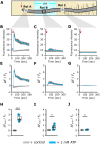
Extracellular ATP (eATP) increases cytosolic free calcium ([Ca2+ ]cyt ) as a specific second messenger 'signature' through the plasma membrane DORN1/P2K1 receptor. Previous studies revealed a biphasic signature in Arabidopsis thaliana roots that is altered by inorganic phosphate (Pi) deprivation. The relationship between the two phases of the signature and possible wave formation have been tested as a function of Pi nutrition. The bioluminescent aequorin and intensiometric GCaMP3 reporters were used to resolve the spatial origin of the eATP [Ca2+ ]cyt signature in Arabidopsis root tips. Application of eATP only to the root apex allowed [Ca2+ ]cyt wave resolution without the confounding effects of eATP delivery by superfusion. The first apical millimetre of the root generates the first [Ca2+ ]cyt increase by eATP, regardless of nutritional status. The second increase occurs sub-apically in the root hair zone, has some autonomy and is significantly reduced in Pi-starved roots. A significant component of the Pi-replete signature does not require DORN1/P2K1, but Pi-starved roots appear to have an absolute requirement for that receptor. Application of eATP specifically to the root apex provides evidence for cell-to-cell propagation of a [Ca2+ ]cyt wave that diminishes sub-apically. The apex maintains a robust [Ca2+ ]cyt increase (even under Pi starvation) that is the basis of a propagative wave, with implications for the ability of the root's eATP signalling systems to signal systemically. Partial autonomy of the sub-apical region may be relevant to the perception of eATP from microbes. eATP-induced [Ca2+ ]cyt increase may not have always have an obligate requirement for DORN1/P2K1.
Keywords: Arabidopsis; calcium; extracellular ATP; phosphate; root; signature; wave.
© 2022 German Society for Plant Sciences and The Royal Botanical Society of the Netherlands.
Figures


References
-
- Chehab E.W., Yao C., Henderson Z., Kim S., Braam J. (2012) Arabidopsis touch‐induced morphogenesis is jasmonate‐mediated and protects against stress. Current Biology, 22, 701–706. - PubMed
-
- Choi J., Tanaka K., Cao Y., Qi Y., Qiu J., Liang Y., Lee S.Y., Stacey G. (2014a) Identification of a plant receptor for extracellular ATP. Science, 343, 290–294. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

