Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity
- PMID: 35395152
- PMCID: PMC9117128
- DOI: 10.1038/s41586-022-04716-y
Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity
Abstract
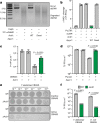
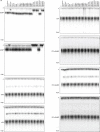
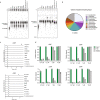
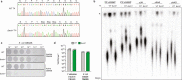
The cyclic oligonucleotide-based antiphage signalling system (CBASS) and the pyrimidine cyclase system for antiphage resistance (Pycsar) are antiphage defence systems in diverse bacteria that use cyclic nucleotide signals to induce cell death and prevent viral propagation1,2. Phages use several strategies to defeat host CRISPR and restriction-modification systems3-10, but no mechanisms are known to evade CBASS and Pycsar immunity. Here we show that phages encode anti-CBASS (Acb) and anti-Pycsar (Apyc) proteins that counteract defence by specifically degrading cyclic nucleotide signals that activate host immunity. Using a biochemical screen of 57 phages in Escherichia coli and Bacillus subtilis, we discover Acb1 from phage T4 and Apyc1 from phage SBSphiJ as founding members of distinct families of immune evasion proteins. Crystal structures of Acb1 in complex with 3'3'-cyclic GMP-AMP define a mechanism of metal-independent hydrolysis 3' of adenosine bases, enabling broad recognition and degradation of cyclic dinucleotide and trinucleotide CBASS signals. Structures of Apyc1 reveal a metal-dependent cyclic NMP phosphodiesterase that uses relaxed specificity to target Pycsar cyclic pyrimidine mononucleotide signals. We show that Acb1 and Apyc1 block downstream effector activation and protect from CBASS and Pycsar defence in vivo. Active Acb1 and Apyc1 enzymes are conserved in phylogenetically diverse phages, demonstrating that cleavage of host cyclic nucleotide signals is a key strategy of immune evasion in phage biology.
© 2022. The Author(s).
Conflict of interest statement
R.S. is a scientific cofounder and advisor of BiomX and Ecophage. The other authors declare no competing interests.
Figures










Comment in
-
Killing the messenger to evade bacterial defences.Nature. 2022 May;605(7910):431-432. doi: 10.1038/d41586-022-01127-x. Nature. 2022. PMID: 35488060 No abstract available.
-
Turning down the (C)BASS: Phage-encoded inhibitors jam bacterial immune signaling.Mol Cell. 2022 Jun 16;82(12):2185-2187. doi: 10.1016/j.molcel.2022.05.030. Mol Cell. 2022. PMID: 35714582
References
-
- Cohen D, et al. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature. 2019;574:691–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

