Validation of a rapid, saliva-based, and ultra-sensitive SARS-CoV-2 screening system for pandemic-scale infection surveillance
- PMID: 35395856
- PMCID: PMC8990279
- DOI: 10.1038/s41598-022-08263-4
Validation of a rapid, saliva-based, and ultra-sensitive SARS-CoV-2 screening system for pandemic-scale infection surveillance
Abstract
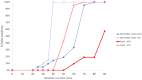
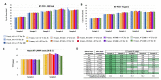
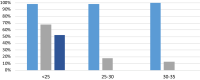
Without any realistic prospect of comprehensive global vaccine coverage and lasting immunity, control of pandemics such as COVID-19 will require implementation of large-scale, rapid identification and isolation of infectious individuals to limit further transmission. Here, we describe an automated, high-throughput integrated screening platform, incorporating saliva-based loop-mediated isothermal amplification (LAMP) technology, that is designed for population-scale sensitive detection of infectious carriers of SARS-CoV-2 RNA. Central to this surveillance system is the "Sentinel" testing instrument, which is capable of reporting results within 25 min of saliva sample collection with a throughput of up to 3840 results per hour. It incorporates continuous flow loading of samples at random intervals to cost-effectively adjust for fluctuations in testing demand. Independent validation of our saliva-based RT-LAMP technology on an automated LAMP instrument coined the "Sentinel", found 98.7% sensitivity, 97.6% specificity, and 98% accuracy against a RT-PCR comparator assay, confirming its suitability for surveillance screening. This Sentinel surveillance system offers a feasible and scalable approach to complement vaccination, to curb the spread of COVID-19 variants, and control future pandemics to save lives.
© 2022. The Author(s).
Conflict of interest statement
PW and PO are founders of Avicena Systems Ltd, SK is founder of Prion Ltd.
Figures

Similar articles
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
A semi-automated, isolation-free, high-throughput SARS-CoV-2 reverse transcriptase (RT) loop-mediated isothermal amplification (LAMP) test.Sci Rep. 2021 Nov 1;11(1):21385. doi: 10.1038/s41598-021-00827-0. Sci Rep. 2021. PMID: 34725400 Free PMC article.
-
A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples.Sci Rep. 2021 Aug 12;11(1):16430. doi: 10.1038/s41598-021-95799-6. Sci Rep. 2021. PMID: 34385527 Free PMC article.
-
CRISPR-based assays for rapid detection of SARS-CoV-2.Methods. 2022 Jul;203:594-603. doi: 10.1016/j.ymeth.2020.10.003. Epub 2020 Oct 10. Methods. 2022. PMID: 33045362 Free PMC article. Review.
-
Diagnostic efficiency of RT-LAMP integrated CRISPR-Cas technique for COVID-19: A systematic review and meta-analysis.Pathog Glob Health. 2022 Oct;116(7):410-420. doi: 10.1080/20477724.2022.2035625. Epub 2022 Feb 10. Pathog Glob Health. 2022. PMID: 35142264 Free PMC article.
Cited by
-
Cross comparison of alternative diagnostic protocols including substitution to the clinical sample, RNA extraction method and nucleic acid amplification technology for COVID-19 diagnosis.Front Mol Biosci. 2024 Aug 23;11:1445142. doi: 10.3389/fmolb.2024.1445142. eCollection 2024. Front Mol Biosci. 2024. PMID: 39247206 Free PMC article.
-
Low rate of SARS-CoV-2 incident infection identified by weekly screening PCR in a prospective year-long cohort study.PLoS One. 2022 Sep 26;17(9):e0274078. doi: 10.1371/journal.pone.0274078. eCollection 2022. PLoS One. 2022. PMID: 36155639 Free PMC article.
-
Saliva as Biomarker for Oral and Chronic Degenerative Non-Communicable Diseases.Metabolites. 2023 Jul 27;13(8):889. doi: 10.3390/metabo13080889. Metabolites. 2023. PMID: 37623833 Free PMC article. Review.
-
Laboratory-based molecular test alternatives to RT-PCR for the diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Oct 14;10(10):CD015618. doi: 10.1002/14651858.CD015618. Cochrane Database Syst Rev. 2024. PMID: 39400904
-
Influenza Virus Genomic Mutations, Host Barrier and Cross-species Transmission.Curr Genomics. 2025;26(3):161-174. doi: 10.2174/0113892029316603240926051325. Epub 2024 Oct 11. Curr Genomics. 2025. PMID: 40433418 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

