Incremental benefits of novel pharmaceuticals in the UK: a cross-sectional analysis of NICE technology appraisals from 2010 to 2020
- PMID: 35396306
- PMCID: PMC8996039
- DOI: 10.1136/bmjopen-2021-058279
Incremental benefits of novel pharmaceuticals in the UK: a cross-sectional analysis of NICE technology appraisals from 2010 to 2020
Abstract
Objectives: To evaluate the incremental value of new drugs across disease areas receiving favourable coverage decisions by the UK's National Institute for Health and Care Excellence (NICE) over the past decade.
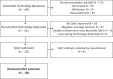
Design, setting, and participants: This cross-sectional study assessed favourable appraisal decisions of drugs between 1 January 2010 and 31 December 2020. Estimates of incremental benefit were extracted from NICE's evidence review groups reports.
Primary outcome measure: Incremental benefit of novel drugs relative to the best alternative therapeutic option, expressed in quality-adjusted life-years (QALYs).
Results: 184 appraisals of 129 drugs provided QALYs. The median incremental value was 0.27 QALY (IQR: 0.07-0.73). Benefits varied across drug-indication pairs (range: -0.49 to 5.22 QALY). The highest median benefits were found in haematology (0.70, IQR: 0.55-1.22) and oncology (0.46, IQR: 0.20-0.88), the lowest in ophthalmology (0.09, IQR: 0.04-0.22) and endocrinology (0.02, IQR: 0.01-0.06). Eight appraisals (4.3%) found contributions of more than two QALYs, but one in four (50/184) drug-indication pairs provided less than the equivalent of 1 month in perfect health compared to existing treatments.
Conclusions: In our review period, the median incremental value of novel drugs approved for use within the English National Health System, relative to the best alternative therapeutic option, was equivalent to 3-4 months of life in perfect health, but data were heterogeneous. Objective evaluations of therapeutic value helps patients and physicians to develop reasonable expectations of drugs and delivers insights into disease areas where medicinal therapeutic progress has had the most and least impact.
Keywords: general medicine (see internal medicine); health policy; therapeutics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: TBP is employed part-time by myTomorrows, which facilitates expanded access programs for the pharmaceutical industry. TBP holds stock in myTomorrows (<0.1%). TBP has received funding for research from the Dutch government (grant EMCLSH20012). The research of TBP is conducted independently and TBP is contractually free to publish any results for all the conducted work. None of the recent work concerns the topic in this analysis. DGJC received speaker fees from Takeda. MMV is director of the institute of Medical Technology Assessment (iMTA). iMTA conducts cost-effectiveness research funded by international governments, pharmaceutical industry, and med-tech industry. All research is conducted independently and iMTA is contractually free to publish any results for all conducted work. None of the recent work concerns the topic in this analysis. JJD receives funding from Arnold Ventures, the Greenwall Foundation, the Kaiser Permanente Institute for Health Policy, West Health, and the Novo Nordisk Foundation (grant for a scientifically independent Collaborative Research Programme; grant NNF17SA0027784).
Figures

Similar articles
-
Population-health impact of new drugs recommended by the National Institute for Health and Care Excellence in England during 2000-20: a retrospective analysis.Lancet. 2025 Jan 4;405(10472):50-60. doi: 10.1016/S0140-6736(24)02352-3. Epub 2024 Dec 12. Lancet. 2025. PMID: 39675371
-
Audit of data redaction practices in NICE technology appraisals from 1999 to 2019.BMJ Open. 2021 Oct 6;11(10):e051812. doi: 10.1136/bmjopen-2021-051812. BMJ Open. 2021. PMID: 34615680 Free PMC article.
-
A Decade of Health Innovation: The Impact of New Medicines on Patient Health and the Implications for NICE's Size of Benefit Multiplier.Value Health. 2023 Oct;26(10):1435-1439. doi: 10.1016/j.jval.2023.06.009. Epub 2023 Jun 29. Value Health. 2023. PMID: 37391164
-
Modelling approaches for histology-independent cancer drugs to inform NICE appraisals: a systematic review and decision-framework.Health Technol Assess. 2021 Dec;25(76):1-228. doi: 10.3310/hta25760. Health Technol Assess. 2021. PMID: 34990339
-
Azacitidine for Treating Acute Myeloid Leukaemia with More Than 30 % Bone Marrow Blasts: An Evidence Review Group Perspective of a National Institute for Health and Care Excellence Single Technology Appraisal.Pharmacoeconomics. 2017 Mar;35(3):363-373. doi: 10.1007/s40273-016-0453-5. Pharmacoeconomics. 2017. PMID: 27752999 Review.
Cited by
-
Benefits of early highly effective versus escalation treatment strategies in relapsing multiple sclerosis estimated using a treatment-sequence model.Mult Scler. 2024 Jul;30(8):1016-1025. doi: 10.1177/13524585241258692. Epub 2024 Jun 10. Mult Scler. 2024. PMID: 38859625 Free PMC article.
-
Cost-effectiveness analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at low risk of surgical mortality in Sweden.Ups J Med Sci. 2024 Nov 7;129. doi: 10.48101/ujms.v129.10741. eCollection 2024. Ups J Med Sci. 2024. PMID: 39691778 Free PMC article.
-
Cost-effectiveness analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement in patients with severe aortic stenosis at low risk of surgical mortality in Sweden.Ups J Med Sci. 2025 Apr 4;130. doi: 10.48101/ujms.v130.10741. eCollection 2025. Ups J Med Sci. 2025. PMID: 40313688 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
