Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation
- PMID: 35396339
- PMCID: PMC9148984
- DOI: 10.1136/jnnp-2021-328391
Aetiology, secondary prevention strategies and outcomes of ischaemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation
Abstract
Objective: To investigate the aetiology, subsequent preventive strategies and outcomes of stroke despite anticoagulation in patients with atrial fibrillation (AF).
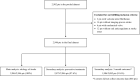
Methods: We analysed consecutive patients with AF with an index imaging-proven ischaemic stroke despite vitamin K-antagonist (VKA) or direct oral anticoagulant (DOAC) treatment across 11 stroke centres. We classified stroke aetiology as: (i) competing stroke mechanism other than AF-related cardioembolism; (ii) insufficient anticoagulation (non-adherence or low anticoagulant activity measured with drug-specific assays); or, (iii) AF-related cardioembolism despite sufficient anticoagulation. We investigated subsequent preventive strategies with regard to the primary (composite of recurrent ischaemic stroke, intracranial haemorrhage, death) and secondary endpoint (recurrent ischaemic stroke) within 3 months after index stroke.
Results: Among 2946 patients (median age 81 years; 48% women; 43% VKA, 57% DOAC), stroke aetiology was competing mechanism in 713 patients (24%), insufficient anticoagulation in 934 (32%) and cardioembolism despite sufficient anticoagulation in 1299 (44%). We found high rates of the primary (27% of patients; completeness 91.6%) and secondary endpoint (4.6%; completeness 88.5%). Only DOAC (vs VKA) treatment after index stroke showed lower odds for both endpoints (primary: adjusted OR (aOR) (95% CI) 0.49 (0.32 to 0.73); secondary: 0.44 (0.24 to 0.80)), but not switching between different DOAC types. Adding antiplatelets showed higher odds for both endpoints (primary: aOR (95% CI) 1.99 (1.25 to 3.15); secondary: 2.66 (1.40 to 5.04)). Only few patients (1%) received left atrial appendage occlusion as additional preventive strategy.
Conclusions: Stroke despite anticoagulation comprises heterogeneous aetiologies and cardioembolism despite sufficient anticoagulation is most common. While DOAC were associated with better outcomes than VKA, adding antiplatelets was linked to worse outcomes in these high-risk patients. Our findings indicate that individualised and novel preventive strategies beyond the currently available anticoagulants are needed.
Trial registration number: ISRCTN48292829.
Keywords: atrial fibrillation; etiology; outcome; prevention strategies; stroke despite anticoagulation.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JFS: grant from Corona-Stiftung, outside the submitted work. CHN: grants from German Ministry of Research and Education, German Center for Neurodegenerative Diseases, German Center for Cardiovascular Research; speaker and/or consultation fees from Bayer, Boehringer Ingelheim, Bristol Myers Squibb (BMS), Pfizer, Alexion, Daiichi-Sankyo, Abbott, W.L. Gore and Associates. CS: departmental funding from Massachusetts General Hospital for the Neuro-AF study. SY: steering committee member Neuro-AF study, non-funded; research collaboration Medtronic, non-funded. KGH: speaker’s honoraria, consulting fees, lecture honoraria and/or grants from Abbott, Alexion, AMARIN, AstraZeneca, Bayer, Sanofi, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, BMS, Biotronik, Medtronic, Premier Research, Portola, W.L. Gore and Associates, Sun Pharma, Edwards Lifesciences. CC: grants from Swiss Heart Foundation (SHF); other support from iSchemaView and Bayer, outside the submitted work. KG: personal fees and/or non-financial support from Alexion Germany GmbH, Abbott Medical, Bayer Vital GmbH, Boehringer Ingelheim, BMS, Daiichi Sankyo, outside the submitted work. MK: funding from Swiss National Science Foundation (SNSF), SHF; contributions from BRAHMS Thermo Fisher, Roche; advisory fees from Bayer, AstraZeneca, Medtronic. SW: research support from SNSF, Olga Mayenfisch Foundation, University of Zurich CRPP stroke; speaker honoraria from Amgen; travel honoraria from Bayer; research grant from Boehringer Ingelheim. STE: research grants from SNSF, SHF, research support from Daiichi Sankyo; educational grant from Pfizer, compensation from Stago for educational material; travel/speaker honoraria from Bayer, Boehringer Ingelheim, BMS, Daiichi Sankyo; advisory board Bayer, Boehringer Ingelheim, BMS. PAR: speaker’s honoraria and lecture fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, outside the submitted work. UF: research support from the SNSF (32003B_197009), SHF, Medtronic; consultant for Medtronic, Stryker, CSL Behring; advisory board Portola/Alexion (money paid to institution). BK: consultant fees and travel expenses from Bayer, Daiichi Sankyo, Pfizer, Medtronic, outside the submitted work. JCP: consultation fees and travel expenses from Akcea, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, outside the submitted work. DJS: advisory board for Bayer Switzerland AG and Portola/Alexion, research funding from SNSF, SHF, Bangerter-Rhyner Foundation, Swiss Society of Neurology, Bayer Foundation. The remaining authors declare no conflicts of interest.
Figures


References
-
- Rost NS, Giugliano RP, Ruff CT, et al. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from engage AF-TIMI 48 (effective anticoagulation with factor Xa next generation in atrial Fibrillation-Thrombolysis in myocardial infarction 48). Stroke 2016;47:2075–82. 10.1161/STROKEAHA.116.013540 - DOI - PubMed
