Prenatal opioid-exposed infant extracellular miRNA signature obtained at birth predicts severity of neonatal opioid withdrawal syndrome
- PMID: 35396369
- PMCID: PMC8993911
- DOI: 10.1038/s41598-022-09793-7
Prenatal opioid-exposed infant extracellular miRNA signature obtained at birth predicts severity of neonatal opioid withdrawal syndrome
Abstract
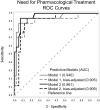
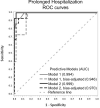
Prenatal opioid exposure (POE) is commonly associated with neonatal opioid withdrawal syndrome (NOWS), which is characterized by a broad variability in symptoms and severity. Currently there are no diagnostic tools to reliably predict which infants will develop severe NOWS, while risk stratification would allow for proactive decisions about appropriate clinical monitoring and interventions. The aim of this prospective cohort study was to assess if extracellular microRNAs (miRNAs) in umbilical cord plasma of infants with POE could predict NOWS severity. Participants (n = 58) consisted of pregnant women receiving medications for opioid use disorder and their infants. NOWS severity was operationalized as the need for pharmacologic treatment and prolonged hospitalization (≥ 14 days). Cord blood miRNAs were assessed using semi-quantitative qRT-PCR arrays. Receiver operating characteristic curves and area under the curve (AUC) were estimated. The expression of three miRNAs (miR-128-3p, miR-30c-5p, miR-421) predicted need for pharmacologic treatment (AUC: 0.85) and prolonged hospitalization (AUC: 0.90). Predictive validity improved after two miRNAs (let-7d-5p, miR-584-5p) were added to the need for pharmacologic treatment model (AUC: 0.94) and another two miRNAs (let-7b-5p, miR-10-5p) to the prolonged hospitalization model (AUC: 0.99). Infant cord blood extracellular miRNAs can proactively identify opioid-exposed neonates at high-risk for developing severe NOWS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Neonatal opioid toxicity: opioid withdrawal (abstinence) syndrome with emphasis on pharmacogenomics and respiratory depression.Arch Toxicol. 2023 Oct;97(10):2575-2585. doi: 10.1007/s00204-023-03563-8. Epub 2023 Aug 3. Arch Toxicol. 2023. PMID: 37537419 Review.
-
Severity of neonatal opioid withdrawal syndrome with prenatal exposure to serotonin reuptake inhibitors.Pediatr Res. 2022 Mar;91(4):867-873. doi: 10.1038/s41390-021-01756-4. Epub 2021 Sep 29. Pediatr Res. 2022. PMID: 34588611 Free PMC article.
-
Reconceptualizing non-pharmacologic approaches to Neonatal Abstinence Syndrome (NAS) and Neonatal Opioid Withdrawal Syndrome (NOWS): A theoretical and evidence-based approach.Neurotoxicol Teratol. 2021 Nov-Dec;88:107020. doi: 10.1016/j.ntt.2021.107020. Epub 2021 Aug 19. Neurotoxicol Teratol. 2021. PMID: 34419619 Free PMC article. Review.
-
Newborn Cry Acoustics in the Assessment of Neonatal Opioid Withdrawal Syndrome Using Machine Learning.JAMA Netw Open. 2022 Oct 3;5(10):e2238783. doi: 10.1001/jamanetworkopen.2022.38783. JAMA Netw Open. 2022. PMID: 36301544 Free PMC article.
-
Reconceptualizing non-pharmacologic approaches to Neonatal Abstinence Syndrome (NAS) and Neonatal Opioid Withdrawal Syndrome (NOWS): A theoretical and evidence-based approach. Part II: The clinical application of nonpharmacologic care for NAS/NOWS.Neurotoxicol Teratol. 2021 Nov-Dec;88:107032. doi: 10.1016/j.ntt.2021.107032. Epub 2021 Sep 29. Neurotoxicol Teratol. 2021. PMID: 34600100 Review.
Cited by
-
Neural crest cells and fetal alcohol spectrum disorders: Mechanisms and potential targets for prevention.Pharmacol Res. 2023 Aug;194:106855. doi: 10.1016/j.phrs.2023.106855. Epub 2023 Jul 17. Pharmacol Res. 2023. PMID: 37460002 Free PMC article. Review.
-
Microbiota and nutrition as risk and resiliency factors following prenatal alcohol exposure.Front Neurosci. 2023 Jun 15;17:1182635. doi: 10.3389/fnins.2023.1182635. eCollection 2023. Front Neurosci. 2023. PMID: 37397440 Free PMC article. Review.
-
Epigenetic Insights into Substance Use Disorder and Associated Psychiatric Conditions.Complex Psychiatry. 2025 Mar 3;11(1):12-36. doi: 10.1159/000544912. eCollection 2025 Jan-Dec. Complex Psychiatry. 2025. PMID: 40201238 Review.
-
Advances in the Care of Infants With Prenatal Opioid Exposure and Neonatal Opioid Withdrawal Syndrome.Pediatrics. 2024 Jan 1;153(2):e2023062871. doi: 10.1542/peds.2023-062871. Pediatrics. 2024. PMID: 38178779 Free PMC article. Review.
-
A review of the genomics of neonatal abstinence syndrome.Front Genet. 2023 Feb 10;14:1140400. doi: 10.3389/fgene.2023.1140400. eCollection 2023. Front Genet. 2023. PMID: 36845389 Free PMC article. Review.
References
-
- National Academies of Sciences Engineering and Medicine (U.S.). Committee on Medication-Assisted Treatment for Opioid Use Disorder. Leshner AI, Mancher M, National Academies of Sciences Engineering and Medicine (U.S.). Board on Health Sciences Policy. & National Academies of Sciences Engineering and Medicine (U.S.). Health and Medicine Division . Medications for Opioid Use Disorder Save Lives. The National Academies Press; 2019. - PubMed
-
- Healthcare Cost and Utilization Project. HCUP Fast Stats—Neonatal Abstinence Syndrome (NAS) Among Newborn Hospitalizations (2021). www.hcup-us.ahrq.gov/faststats/nas/nasquery.jsp?setting1=IP&location=US. Accessed 12 April 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

