Dopamine receptor 1 expressing B cells exert a proinflammatory role in female patients with rheumatoid arthritis
- PMID: 35396380
- PMCID: PMC8993840
- DOI: 10.1038/s41598-022-09891-6
Dopamine receptor 1 expressing B cells exert a proinflammatory role in female patients with rheumatoid arthritis
Abstract
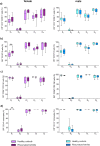
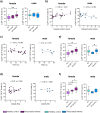
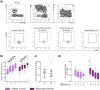
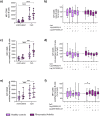
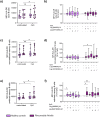
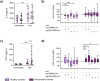
Rheumatoid arthritis (RA) is a chronic rheumatic disease with a clear sex-bias. Recent data indicated a role for dopamine in RA pathogenesis, while dopaminergic pathways can be modulated by estrogens. As defined mechanism of action of dopamine on B cell function in RA are unclear, we aimed to elucidate this, with special focus on sex-differences. Healthy controls (HC, n = 64) and RA patients (n = 61) were recruited. Expression of D1-D5 dopamine receptors (DRs) was investigated by flow cytometry on peripheral blood mononuclear cells (PBMCs). D1-like DRs were stimulated in vitro to assess effects on B cell activation and proliferation. Secretion of cytokines and dopamine content were measured by ELISA. All DRs were expressed on PBMCs of HC and RA patients. Dopamine content in PBMCs, and frequency of D1DR expressing B cells were significantly higher in RA females (p < 0.001). Expression of D1DR on RA B cells correlated positively with disease duration and severity only in women. Combined B cell and D1-like DR stimulation induced higher IL-8 and CCL-3 secretion from PBMCs of female RA patients compared to HC. These results indicate sex-specific differences in dopaminergic pathway in RA, with a proinflammatory feature of the D1DR pathway in women.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

