The role of sphingosine-1-phosphate in bone remodeling and osteoporosis
- PMID: 35396384
- PMCID: PMC8993882
- DOI: 10.1038/s41413-022-00205-0
The role of sphingosine-1-phosphate in bone remodeling and osteoporosis
Abstract
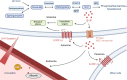
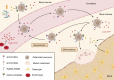
Osteoporosis is a systemic bone disease that affects more than 200 million people worldwide and is caused by the disruption of the equilibrium between osteoclastic bone resorption and osteoblastic bone formation. Sphingosine-1-phosphate (S1P) is a natural, bioactive sphingolipid that has been shown to play a major role in cardiovascular and immunological pathologies by regulating biological and cellular processes, including migration, differentiation, proliferation and survival. Recent studies also suggest a central role for S1P in bone diseases, including osteoporosis; however, the effects of S1P, particularly in bone metabolism, remain to be further elucidated. In this review, we summarize the available literature on the role of S1P in bone metabolism with a focus on osteoporosis. On the cellular level, S1P acts as an osteoclast-osteoblast coupling factor to promote osteoblast proliferation and bone formation. Moreover, the recruitment of osteoclast precursors to resorption sites is regulated by the interplay of S1P gradients and S1P receptor expression. From a clinical perspective, increasing evidence suggests that systemically elevated S1P blood levels may serve as an independent risk factor for osteoporosis-related fractures. Taken together, S1P signaling is a potential therapeutic target and may serve as a novel biomarker in patients with systemic bone disease.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

