HIF-1α-regulated lncRNA-TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction
- PMID: 35396503
- PMCID: PMC8993815
- DOI: 10.1038/s41420-022-00969-8
HIF-1α-regulated lncRNA-TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction
Abstract
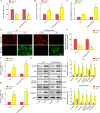
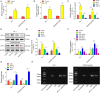
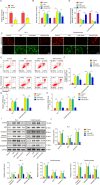
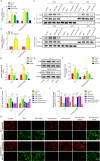
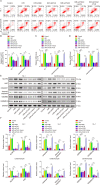
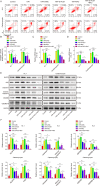
Myocardial infarction (MI) is a fatal heart disease that affects millions of lives worldwide each year. This study investigated the roles of HIF-1α/lncRNA-TUG1 in mitochondrial dysfunction and pyroptosis in MI. CCK-8, DHE, lactate dehydrogenase (LDH) assays, and JC-1 staining were performed to measure proliferation, reactive oxygen species (ROS), LDH leakage, and mitochondrial damage in hypoxia/reoxygenation (H/R)-treated cardiomyocytes. Enzyme-linked immunoassay (ELISA) and flow cytometry were used to detect LDH, creatine kinase (CK), and its isoenzyme (CK-MB) levels and caspase-1 activity. Chromatin immunoprecipitation (ChIP), luciferase assay, and RNA-immunoprecipitation (RIP) were used to assess the interaction between HIF-1α, TUG1, and FUS. Quantitative real-time polymerase chain reaction (qRT-PCR), Western blotting, and immunohistochemistry were used to measure HIF-1α, TUG1 and pyroptosis-related molecules. Hematoxylin and eosin (HE), 2,3,5-triphenyltetrazolium chloride (TTC), and terminal deoxynucleotidyl transferase dUTP risk end labelling (TUNEL) staining were employed to examine the morphology, infarction area, and myocardial injury in the MI mouse model. Mitochondrial dysfunction and pyroptosis were induced in H/R-treated cardiomyocytes, accompanied by an increase in the expression of HIF-α and TUG1. HIF-1α promoted TUG1 expression by directly binding to the TUG1 promoter. TUG1 silencing inhibited H/R-induced ROS production, mitochondrial injury and the expression of the pyroptosis-related proteins NLRP3, caspase-1 and GSDMD. Additionally, H/R elevated FUS levels in cardiomyocytes, which were directly inhibited by TUG1 silencing. Fused in sarcoma (FUS) overexpression reversed the effect of TUG1 silencing on mitochondrial damage and caspase-1 activation. However, the ROS inhibitor N-acetylcysteine (NAC) promoted the protective effect of TUG1 knockdown on H/R-induced cardiomyocyte damage. The in vivo MI model showed increased infarction, myocardial injury, ROS levels and pyroptosis, which were inhibited by TUG1 silencing. HIF-1α targeting upregulated TUG1 promotes mitochondrial damage and cardiomyocyte pyroptosis by combining with FUS, thereby promoting the occurrence of MI. HIF-1α/TUG1/FUS may serve as a potential treatment target for MI.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Mechanic OJ, Grossman SA. Acute Myocardial Infarction. StatPearls. Treasure Island (FL); 2020.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

