Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip
- PMID: 35396513
- PMCID: PMC8993817
- DOI: 10.1038/s41467-022-29562-4
Mechanical control of innate immune responses against viral infection revealed in a human lung alveolus chip
Abstract
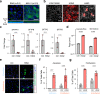
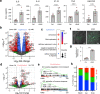
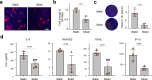
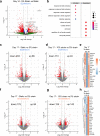
Mechanical breathing motions have a fundamental function in lung development and disease, but little is known about how they contribute to host innate immunity. Here we use a human lung alveolus chip that experiences cyclic breathing-like deformations to investigate whether physical forces influence innate immune responses to viral infection. Influenza H3N2 infection of mechanically active chips induces a cascade of host responses including increased lung permeability, apoptosis, cell regeneration, cytokines production, and recruitment of circulating immune cells. Comparison with static chips reveals that breathing motions suppress viral replication by activating protective innate immune responses in epithelial and endothelial cells, which are mediated in part through activation of the mechanosensitive ion channel TRPV4 and signaling via receptor for advanced glycation end products (RAGE). RAGE inhibitors suppress cytokines induction, while TRPV4 inhibition attenuates both inflammation and viral burden, in infected chips with breathing motions. Therefore, TRPV4 and RAGE may serve as new targets for therapeutic intervention in patients infected with influenza and other potential pandemic viruses that cause life-threatening lung inflammation.
© 2022. The Author(s).
Conflict of interest statement
D.E.I. is a founder, board member, SAB chair, and holds equity in Emulate Inc.; D.E.I., H.B. L.S., R.P., and A.J. are inventors on relevant patent applications hold by Harvard University (US 63/135,834 & US 63/187,498). The other authors declare no competing interests.
Figures


Similar articles
-
A(H7N9) virus results in early induction of proinflammatory cytokine responses in both human lung epithelial and endothelial cells and shows increased human adaptation compared with avian H5N1 virus.J Virol. 2015 Apr;89(8):4655-67. doi: 10.1128/JVI.03095-14. Epub 2015 Feb 11. J Virol. 2015. PMID: 25673714 Free PMC article.
-
Differential responses of innate immunity triggered by different subtypes of influenza a viruses in human and avian hosts.BMC Med Genomics. 2017 Dec 21;10(Suppl 4):70. doi: 10.1186/s12920-017-0304-z. BMC Med Genomics. 2017. PMID: 29322931 Free PMC article.
-
Differential Modulation of Innate Immune Responses in Human Primary Cells by Influenza A Viruses Carrying Human or Avian Nonstructural Protein 1.J Virol. 2019 Dec 12;94(1):e00999-19. doi: 10.1128/JVI.00999-19. Print 2019 Dec 12. J Virol. 2019. PMID: 31597767 Free PMC article.
-
TRPV4-A Missing Link Between Mechanosensation and Immunity.Front Immunol. 2020 Mar 10;11:413. doi: 10.3389/fimmu.2020.00413. eCollection 2020. Front Immunol. 2020. PMID: 32210976 Free PMC article. Review.
-
The Potential of Receptor for Advanced Glycation End Products (RAGE) as a Therapeutic Target for Lung Associated Diseases.Curr Drug Targets. 2019;20(6):679-689. doi: 10.2174/1389450120666181120102159. Curr Drug Targets. 2019. PMID: 30457049 Review.
Cited by
-
A Ventilated Three-Dimensional Artificial Lung System for Human Inhalation Exposure Studies.Environ Sci Technol. 2024 Dec 31;58(52):22919-22929. doi: 10.1021/acs.est.4c08315. Epub 2024 Dec 16. Environ Sci Technol. 2024. PMID: 39681299 Free PMC article.
-
Deciphering respiratory viral infections by harnessing organ-on-chip technology to explore the gut-lung axis.Open Biol. 2025 Mar;15(3):240231. doi: 10.1098/rsob.240231. Epub 2025 Mar 5. Open Biol. 2025. PMID: 40037530 Free PMC article. Review.
-
Animal Model Alternatives in Filovirus and Bornavirus Research.Viruses. 2023 Jan 4;15(1):158. doi: 10.3390/v15010158. Viruses. 2023. PMID: 36680198 Free PMC article. Review.
-
Obesity and the Importance of Breathing.Cureus. 2025 Jan 14;17(1):e77431. doi: 10.7759/cureus.77431. eCollection 2025 Jan. Cureus. 2025. PMID: 39811724 Free PMC article. Review.
-
Identification of Critical Genes and Pathways for Influenza A Virus Infections via Bioinformatics Analysis.Viruses. 2022 Jul 26;14(8):1625. doi: 10.3390/v14081625. Viruses. 2022. PMID: 35893690 Free PMC article.
References
-
- Hannezo E, Heisenberg CP. Mechanochemical feedback loops in development and disease. Cell. 2019;178:12–25. - PubMed
-
- Li J, et al. The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev. Cell. 2018;44:297–312.e295. - PubMed
-
- Lopez-Alonso, I. et al. Preventing loss of mechanosensation by the nuclear membranes of alveolar cells reduces lung injury in mice during mechanical ventilation. Sci. Transl. Med.10.1126/scitranslmed.aam7598 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

