Spontaneous Clearance of Vertically Acquired Hepatitis C Infection: Implications for Testing and Treatment
- PMID: 35396848
- PMCID: PMC9989140
- DOI: 10.1093/cid/ciac255
Spontaneous Clearance of Vertically Acquired Hepatitis C Infection: Implications for Testing and Treatment
Abstract
Background: Current guidelines recommend that infants born to women with hepatitis C virus (HCV) viremia be screened for HCV antibody at age 18 months and, if positive, referred for RNA testing at 3 years to confirm chronic infection. This policy is based, in part, on analyses that suggest that 25%-40% of vertically acquired HCV infections clear spontaneously within 4-5 years.
Methods: Data on 179 infants with HCV RNA and/or anti-HCV evidence of vertically acquired infection in 3 prospective European cohorts were investigated. Ages at clearance of infection were estimated taking account of interval censoring and delayed entry. We also investigated clearance in initially HCV RNA-negative infants in whom RNA was not detectable until after 6 weeks.
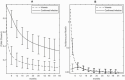
Results: Clearance rates were initially high then declined slowly. Apparently, many infections clear before they can be confirmed. An estimated 65.9% (95% credible interval [CrI], 50.1-81.6) of confirmed infections cleared by 5 years, at a median 12.4 (CrI, 7.1-18.9) months. If treatment were to begin at age 6 months, 18 months, or 3 years, at least 59.0% (CrI, 42.0-76.9), 39.7% (CrI, 17.9-65.9), and 20.9% (CrI, 4.6-44.8) of those treated would clear without treatment. In 7 (6.6%) confirmed infections, RNA was not detectable until after 6 weeks and not until after 6 months in 2 (1.9%). However, all such cases subsequently cleared.
Conclusions: Most confirmed infection cleared by age 3 years. Treatment before age 3, if it was available, would avoid loss to follow-up but would result in substantial overtreatment.
Keywords: HCV; hepatitis C virus; overtreatment; spontaneous clearance; vertical transmission.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. T. is a member of the Infectious Disease in Pregnancy Screening Programme Advisory Board, Public Health England, and reports grants or contracts from the European Commission, Child Health Charitable Incorporated Organisation, Public Health England, Penta Foundation, and ViiV Healthcare via the Penta Foundation, all paid to the institution outside of the submitted work, and payment or honoraria from ViiV Healthcare paid to self. E. C. reports grants or contracts from ViiV Healthcare to the institution via the Penta Foundation outside of the submitted work. I. J. C. reports a Medical Research Council Global Health Trials Development grant and Gilead HCV Elimination competitive grant, both paid to the institution and outside of the submitted work. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Comment in
-
Elimination Means Everyone: Targeting Hepatitis C in Infants and Pregnant Patients.Clin Infect Dis. 2023 Mar 4;76(5):920-922. doi: 10.1093/cid/ciac330. Clin Infect Dis. 2023. PMID: 35475916 No abstract available.
References
-
- Barritt AS, Jhaveri R.. Treatment of hepatitis C during pregnancy—weighing the risks and benefits in contrast to HIV. Curr HIV/AIDS Rep 2018; 15:155–61. - PubMed
-
- Indolfi G, Easterbrook P, Dusheiko G, et al. Hepatitis C virus infection in children and adolescents. The Lancet Gastroenterology & Hepatology 2019; 4:477–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical