Dihydropyrimidine Dehydrogenase Phenotyping Using Pretreatment Uracil: A Note of Caution Based on a Large Prospective Clinical Study
- PMID: 35397172
- PMCID: PMC9322339
- DOI: 10.1002/cpt.2608
Dihydropyrimidine Dehydrogenase Phenotyping Using Pretreatment Uracil: A Note of Caution Based on a Large Prospective Clinical Study
Abstract
In clinical practice, 25-30% of the patients treated with fluoropyrimidines experience severe fluoropyrimidine-related toxicity. Extensively clinically validated DPYD genotyping tests are available to identify patients at risk of severe toxicity due to decreased activity of dihydropyrimidine dehydrogenase (DPD), the rate limiting enzyme in fluoropyrimidine metabolism. In April 2020, the European Medicines Agency recommended that, as an alternative for DPYD genotype-based testing for DPD deficiency, also phenotype testing based on pretreatment plasma uracil levels is a suitable method to identify patients with DPD deficiency. Although the evidence for genotype-directed dosing of fluoropyrimidines is substantial, the level of evidence supporting plasma uracil levels to predict DPD activity in clinical practice is limited. Notwithstanding this, uracil-based phenotyping is now used in clinical practice in various countries in Europe. We aimed to determine the value of pretreatment uracil levels in predicting DPD deficiency and severe treatment-related toxicity. To this end, we determined pretreatment uracil levels in 955 patients with cancer, and assessed the correlation with DPD activity in peripheral blood mononuclear cells (PBMCs) and fluoropyrimidine-related severe toxicity. We identified substantial issues concerning the use of pretreatment uracil in clinical practice, including large between-center study differences in measured pretreatment uracil levels, most likely as a result of pre-analytical factors. Importantly, we were not able to correlate pretreatment uracil levels with DPD activity nor were uracil levels predictive of severe treatment-related toxicity. We urge that robust clinical validation should first be performed before pretreatment plasma uracil levels are used in clinical practice as part of a dosing strategy for fluoropyrimidines.
© 2022 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.H.M.S. and J.H.B. are (part time) employees, stock‐, and patent holders of Modra Pharmaceuticals, a spin out company developing oral taxane formulations; J.H.M.S. is also part time employee of Byondis bv and received consultancy fees from Debiopharm all not related to the contents of the manuscript. D.M. is a current full‐time employee, and shareholder of AstraZeneca, not related to the contents of the manuscript. All other authors declared no competing interests for this work.
Figures
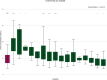
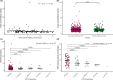
Comment in
-
Response to "Plasma Uracil as a DPD Phenotyping Test: Pre-analytical Handling Matters".Clin Pharmacol Ther. 2023 Mar;113(3):473-475. doi: 10.1002/cpt.2775. Epub 2022 Nov 9. Clin Pharmacol Ther. 2023. PMID: 36352517 No abstract available.
-
Plasma Uracil as a DPD Phenotyping Test: Pre-Analytical Handling Matters!Clin Pharmacol Ther. 2023 Mar;113(3):471-472. doi: 10.1002/cpt.2772. Epub 2022 Nov 22. Clin Pharmacol Ther. 2023. PMID: 36412238 No abstract available.
References
-
- Meulendijks, D. et al. Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine‐associated toxicity: a systematic review and meta‐analysis of individual patient data. Lancet Oncol. 16, 1639–1650 (2015). - PubMed
-
- Hoff, P.M. et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first‐line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J. Clin. Oncol. 19, 2282–2292 (2001). - PubMed
-
- Koopman, M. et al. Sequential versus combination chemotherapy with capecitabine, irinotecan, and oxaliplatin in advanced colorectal cancer (CAIRO): a phase III randomised controlled trial. Lancet 370, 135–142 (2007). - PubMed
-
- Van Cutsem, E. et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III study. J. Clin. Oncol. 19, 4097–4106 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

