Estrogen receptors observed at extranuclear neuronal sites and in glia in the nucleus accumbens core and shell of the female rat: Evidence for localization to catecholaminergic and GABAergic neurons
- PMID: 35397175
- PMCID: PMC9167786
- DOI: 10.1002/cne.25320
Estrogen receptors observed at extranuclear neuronal sites and in glia in the nucleus accumbens core and shell of the female rat: Evidence for localization to catecholaminergic and GABAergic neurons
Abstract
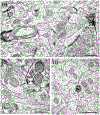
Estrogens affect dopamine-dependent diseases/behavior and have rapid effects on dopamine release and receptor availability in the nucleus accumbens (NAc). Low levels of nuclear estrogen receptor (ER) α and ERβ are seen in the NAc, which cannot account for the rapid effects of estrogens in this region. G-protein coupled ER 1 (GPER1) is observed at low levels in the NAc shell, which also likely does not account for the array of estrogens' effects in this region. Prior studies demonstrated membrane-associated ERs in the dorsal striatum; these experiments extend those findings to the NAc core and shell. Single- and dual-immunolabeling electron microscopy determined whether ERα, ERβ, and GPER1 are at extranuclear sites in the NAc core and shell and whether ERα and GPER1 were localized to catecholaminergic or γ-aminobutyric acid-ergic (GABAergic) neurons. All three ERs are observed, almost exclusively, at extranuclear sites in the NAc, and similarly distributed in the core and shell. ERα, ERβ, and GPER1 are primarily in axons and axon terminals suggesting that estrogens affect transmission in the NAc via presynaptic mechanisms. About 10% of these receptors are found on glia. A small proportion of ERα and GPER1 are localized to catecholaminergic terminals, suggesting that binding at these ERs alters release of catecholamines, including dopamine. A larger proportion of ERα and GPER1 are localized to GABAergic dendrites and terminals, suggesting that estrogens alter GABAergic transmission to indirectly affect dopamine transmission in the NAc. Thus, the localization of ERs could account for the rapid effects of estrogen in the NAc.
Keywords: G-protein coupled estrogen receptor 1; electron microscopy; estrogen receptor alpha; estrogen receptor beta; ventral striatum; γ-aminobutyric acid.
© 2022 Wiley Periodicals LLC.
Figures





Similar articles
-
Estrogen receptor α and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum.Neurosci Lett. 2016 May 27;622:118-23. doi: 10.1016/j.neulet.2016.04.023. Epub 2016 Apr 11. Neurosci Lett. 2016. PMID: 27080432 Free PMC article.
-
Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization.Endocrinology. 2012 Nov;153(11):5373-83. doi: 10.1210/en.2012-1458. Epub 2012 Aug 23. Endocrinology. 2012. PMID: 22919059 Free PMC article.
-
Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females.Horm Behav. 2015 Aug;74:125-38. doi: 10.1016/j.yhbeh.2015.06.010. Epub 2015 Jun 27. Horm Behav. 2015. PMID: 26122294 Free PMC article. Review.
-
Estrogen receptor alpha, G-protein coupled estrogen receptor 1, and aromatase: Developmental, sex, and region-specific differences across the rat caudate-putamen, nucleus accumbens core and shell.J Comp Neurol. 2021 Mar;529(4):786-801. doi: 10.1002/cne.24978. Epub 2020 Aug 3. J Comp Neurol. 2021. PMID: 32632943 Free PMC article.
-
Estrogen signaling: a subtle balance between ER alpha and ER beta.Mol Interv. 2003 Aug;3(5):281-92. doi: 10.1124/mi.3.5.281. Mol Interv. 2003. PMID: 14993442 Review.
Cited by
-
Estrous cycle impacts on dendritic spine plasticity in rat nucleus accumbens core and shell and caudate-putamen.J Comp Neurol. 2023 May;531(7):759-774. doi: 10.1002/cne.25460. Epub 2023 Feb 9. J Comp Neurol. 2023. PMID: 36756791 Free PMC article.
-
Sex and estradiol effects in the rodent dorsal striatum.Eur J Neurosci. 2024 Dec;60(12):6962-6986. doi: 10.1111/ejn.16607. Epub 2024 Nov 21. Eur J Neurosci. 2024. PMID: 39573926 Free PMC article. Review.
-
The importance of translationally evaluating steroid hormone contributions to substance use.Front Neuroendocrinol. 2023 Apr;69:101059. doi: 10.1016/j.yfrne.2023.101059. Epub 2023 Feb 7. Front Neuroendocrinol. 2023. PMID: 36758769 Free PMC article. Review.
-
The impact of estradiol on serotonin, glutamate, and dopamine systems.Front Neurosci. 2024 Mar 22;18:1348551. doi: 10.3389/fnins.2024.1348551. eCollection 2024. Front Neurosci. 2024. PMID: 38586193 Free PMC article. Review.
-
Downregulation of striatal CaV1.3 inhibits the escalation of levodopa-induced dyskinesia in male and female parkinsonian rats of advanced age.Neurobiol Dis. 2023 Jun 1;181:106111. doi: 10.1016/j.nbd.2023.106111. Epub 2023 Mar 29. Neurobiol Dis. 2023. PMID: 37001610 Free PMC article.
References
-
- Almey A, Filardo EJ, Milner TA, & Brake WG (2012). Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: Evidence for cholinergic but not dopaminergic colocalization. Endocrinology, 153(11), 5373–5383. 10.1210/en.2012-1458 - DOI - PMC - PubMed
-
- Alonso-Caraballo Y, & Ferrario CR (2019). Effects of the estrous cycle and ovarian hormones on cue-triggered motivation and intrinsic excitability of medium spiny neurons in the nucleus accumbens core of female rats. Hormones and Behavior, 116, 104583. 10.1016/J.YHBEH.2019.104583 - DOI - PMC - PubMed
-
- Alves SE, Weiland NG, Hayashi S, & McEwen BS (1998). Immunocytochemical localization of nuclear estrogen receptors and progestin receptors within the rat dorsal raphe nucleus. The Journal of Comparative Neurology, 391(3), 322–334. http://www.ncbi.nlm.nih.gov/pubmed/9492203 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

