Epigenetic quantification of immunosenescent CD8+ TEMRA cells in human blood
- PMID: 35397197
- PMCID: PMC9124311
- DOI: 10.1111/acel.13607
Epigenetic quantification of immunosenescent CD8+ TEMRA cells in human blood
Abstract
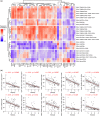
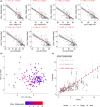
Age-related changes in human T-cell populations are important contributors to immunosenescence. In particular, terminally differentiated CD8+ effector memory CD45RA+ TEMRA cells and their subsets have characteristics of cellular senescence, accumulate in older individuals, and are increased in age-related chronic inflammatory diseases. In a detailed T-cell profiling among individuals over 65 years of age, we found a high interindividual variation among CD8+ TEMRA populations. CD8+ TEMRA proportions correlated positively with cytomegalovirus (CMV) antibody levels, however, not with the chronological age. In the analysis of over 90 inflammation proteins, we identified plasma TRANCE/RANKL levels to associate with several differentiated T-cell populations, including CD8+ TEMRA and its CD28- subsets. Given the strong potential of CD8+ TEMRA cells as a biomarker for immunosenescence, we used deep-amplicon bisulfite sequencing to match their frequencies in flow cytometry with CpG site methylation levels and developed a computational model to predict CD8+ TEMRA cell proportions from whole blood genomic DNA. Our findings confirm the association of CD8+ TEMRA and its subsets with CMV infection and provide a novel tool for their high throughput epigenetic quantification as a biomarker of immunosenescence.
Keywords: CD8+ T-cells; CMV; biomarkers; epigenetics; human aging; inflammation.
© 2022 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Alpert, A. , Pickman, Y. , Leipold, M. , Rosenberg‐Hasson, Y. , Ji, X. , Gaujoux, R. , Rabani, H. , Starosvetsky, E. , Kveler, K. , Schaffert, S. , Furman, D. , Caspi, O. , Rosenschein, U. , Khatri, P. , Dekker, C. L. , Maecker, H. T. , Davis, M. M. , & Shen‐Orr, S. S. (2019). A clinically meaningful metric of immune age derived from high‐dimensional longitudinal monitoring. Nature Medicine, 25(3), 487–495. 10.1038/s41591-019-0381-y - DOI - PMC - PubMed
-
- Appay, V. , Dunbar, P. R. , Callan, M. , Klenerman, P. , Gillespie, G. M. A. , Papagno, L. , Ogg, G. S. , King, A. , Lechner, F. , Spina, C. A. , Little, S. , Havlir, D. V. , Richman, D. D. , Gruener, N. , Pape, G. , Waters, A. , Easterbrook, P. , Salio, M. , Cerundolo, V. , … Rowland‐Jones, S. L. (2002). Memory CD8+ T‐cells vary in differentiation phenotype in different persistent virus infections. Nature Medicine, 8(4), 379–385. 10.1038/nm0402-379 - DOI - PubMed
-
- Baron, U. , Werner, J. , Schildknecht, K. , Schulze, J. J. , Mulu, A. , Liebert, U.‐G. , Sack, U. , Speckmann, C. , Gossen, M. , Wong, R. J. , Stevenson, D. K. , Babel, N. , Schürmann, D. , Baldinger, T. , Bacchetta, R. , Grützkau, A. , Borte, S. , & Olek, S. (2018). Epigenetic immune cell counting in human blood samples for immunodiagnostics. Science Translational Medicine, 10(452). 10.1126/scitranslmed.aan3508 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

