Platelet-leukocyte crosstalk in COVID-19: How might the reciprocal links between thrombotic events and inflammatory state affect treatment strategies and disease prognosis?
- PMID: 35397313
- PMCID: PMC8969450
- DOI: 10.1016/j.thromres.2022.03.022
Platelet-leukocyte crosstalk in COVID-19: How might the reciprocal links between thrombotic events and inflammatory state affect treatment strategies and disease prognosis?
Abstract
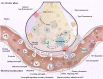
Platelet-leukocyte crosstalk is commonly manifested by reciprocal links between thrombosis and inflammation. Platelet thrombus acts as a reactive matrix that recruits leukocytes to the injury site where their massive accumulation, activation and migration promote thrombotic events while triggering inflammatory responses. As a life-threatening condition with the associations between inflammation and thrombosis, COVID-19 presents diffuse alveolar damage due to exaggerated macrophage activity and cytokine storms. These events, together with direct intracellular virus invasion lead to pulmonary vascular endothelialitis, cell membranes disruption, severe endothelial injury, and thrombosis. The developing pre-alveolar thrombus provides a hyper-reactive milieu that recruits circulating leukocytes to the injury site where their activation contributes to thrombus stabilization and thrombosis propagation, primarily through the formation of Neutrophil extracellular trap (NET). NET fragments can also circulate and deposit in further distance where they may disseminate intravascular thrombosis in severe cases of disease. Thrombi may also facilitate leukocytes migration into alveoli where their accumulation and activation exacerbate cytokine storms and tissue damage, further complicating the disease. Based on these mechanisms, whether an effective anti-inflammatory protocol can prevent thrombotic events, or on the other hand; efficient antiplatelet or anticoagulant regimens may be associated with reduced cytokine storms and tissue damage, is now of interests for several ongoing researches. Thus shedding more light on platelet-leukocyte crosstalk, the review presented here discusses the detailed mechanisms by which platelets may contribute to the pathogenesis of COVID-19, especially in severe cases where their interaction with leukocytes can intensify both inflammatory state and thrombosis in a reciprocal manner.
Keywords: Anti-inflammatory agents; Antiplatelet drugs; COVID-19; Cytokine storms; Damage-associated molecular pattern molecules; Leukocyte Migration; NETs; Platelets; Thrombosis.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Dengue virus infection: how platelet-leukocyte crosstalk shapes thrombotic events and inflammation.Mol Biol Rep. 2025 Jan 13;52(1):119. doi: 10.1007/s11033-025-10222-x. Mol Biol Rep. 2025. PMID: 39804486 Review.
-
Intravascular leukocyte migration through platelet thrombi: directing leukocytes to sites of vascular injury.Thromb Haemost. 2015 Jun;113(6):1224-35. doi: 10.1160/TH14-08-0662. Epub 2015 Apr 16. Thromb Haemost. 2015. PMID: 25880990 Review.
-
Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state.Thromb Res. 2013 Mar;131(3):191-7. doi: 10.1016/j.thromres.2012.11.028. Epub 2012 Dec 20. Thromb Res. 2013. PMID: 23260445 Review.
-
IL-1 induces throboxane-A2 (TxA2) in COVID-19 causing inflammation and micro-thrombi: inhibitory effect of the IL-1 receptor antagonist (IL-1Ra).J Biol Regul Homeost Agents. 2020 Sep-Oct,;34(5):1623-1627. doi: 10.23812/20-34-4EDIT-65. J Biol Regul Homeost Agents. 2020. PMID: 32744052
-
Platelets in the NETworks interweaving inflammation and thrombosis.Front Immunol. 2022 Aug 1;13:953129. doi: 10.3389/fimmu.2022.953129. eCollection 2022. Front Immunol. 2022. PMID: 35979369 Free PMC article. Review.
Cited by
-
Genetic variants in the NF-κB signaling pathway (NFKB1, NFKBIA, NFKBIZ) and risk of critical outcome among COVID-19 patients.Hum Immunol. 2022 Aug-Sep;83(8-9):613-617. doi: 10.1016/j.humimm.2022.06.002. Epub 2022 Jun 21. Hum Immunol. 2022. PMID: 35777990 Free PMC article.
-
Prospective analysis of pre and postoperative laboratory parameters associated with thrombosis in patients with ovarian cancer.J Thromb Thrombolysis. 2024 Mar;57(3):492-496. doi: 10.1007/s11239-023-02944-1. Epub 2024 Jan 28. J Thromb Thrombolysis. 2024. PMID: 38281230
-
In Situ Endothelial SARS-CoV-2 Presence and PROS1 Plasma Levels Alteration in SARS-CoV-2-Associated Coagulopathies.Life (Basel). 2024 Feb 8;14(2):237. doi: 10.3390/life14020237. Life (Basel). 2024. PMID: 38398746 Free PMC article.
-
Platelets and the Atherosclerotic Process: An Overview of New Markers of Platelet Activation and Reactivity, and Their Implications in Primary and Secondary Prevention.J Clin Med. 2023 Sep 20;12(18):6074. doi: 10.3390/jcm12186074. J Clin Med. 2023. PMID: 37763014 Free PMC article. Review.
-
Risk of Venous Thromboembolism in Multiple Myeloma Patients During the Immediate Peri-Autologous Hematopoietic Cell Transplantation Phase.Clin Appl Thromb Hemost. 2023 Jan-Dec;29:10760296231177678. doi: 10.1177/10760296231177678. Clin Appl Thromb Hemost. 2023. PMID: 37277999 Free PMC article.
References
-
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

