The SARS-CoV-2 spike residues 616/644 and 1138/1169 delineate two antibody epitopes in COVID-19 mRNA COMINARTY vaccine (Pfizer/BioNTech)
- PMID: 35397679
- PMCID: PMC8994064
- DOI: 10.1038/s41598-022-10057-7
The SARS-CoV-2 spike residues 616/644 and 1138/1169 delineate two antibody epitopes in COVID-19 mRNA COMINARTY vaccine (Pfizer/BioNTech)
Erratum in
-
Author Correction: The SARS-CoV-2 spike residues 616/644 and 1138/1169 delineate two antibody epitopes in COVID-19 mRNA COMIRNATY vaccine (Pfizer/BioNTech).Sci Rep. 2022 Apr 20;12(1):6498. doi: 10.1038/s41598-022-10885-7. Sci Rep. 2022. PMID: 35444227 Free PMC article. No abstract available.
Abstract
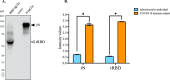
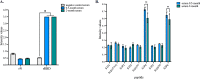
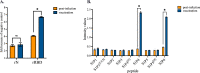
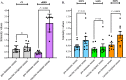
The newly identified coronavirus SARS-CoV-2 is responsible for the worldwide pandemic COVID-19. Considerable efforts have been devoted for the development of effective vaccine strategies against COVID-19. The SARS-CoV-2 spike protein has been identified as the major antigen candidate for the development of COVID-19 vaccines. The Pfizer-BioNTech COVID-19 vaccine COMIRNATY is a lipid nanoparticle-encapsulated mRNA encoding a full-length and prefusion-stabilized SARS-CoV-2 spike protein. In the present study, synthetic peptide-based ELISA assays were performed to identify linear B-cell epitopes into the spike protein that contribute to elicitation of antibody response in COMIRNATY-vaccinated individuals. The synthetic S2P6 peptide containing the spike residues 1138/1169 and to a lesser extent, the synthetic S1P4 peptide containing the spike residues 616/644 were recognized by the immune sera from COMIRNATY vaccine recipients but not COVID-19 recovered patients. We assume that the synthetic S2P6 peptide and to a lesser extent the synthetic S1P4 peptide, could be of interest to measure the dynamic of antibody response to COVID-19 mRNA vaccines. The S2P6 peptide has been identified as immunogenic in adult BALB/c mice that received protein-peptide conjugates in a prime-boost schedule. This raises the question on the role of the B-cell epitope peptide containing the SARS-CoV-2 spike residues 1138/1169 in protective efficacy of the Pfizer-BioNTech COVID-19 vaccine COMIRNATY.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

