Should routine risk reduction procedures for the prevention and control of pandemics become a standard in all oncological outpatient clinics? The prospective COVID-19 cohort study: protect-CoV
- PMID: 35397689
- PMCID: PMC8994860
- DOI: 10.1007/s12032-022-01700-4
Should routine risk reduction procedures for the prevention and control of pandemics become a standard in all oncological outpatient clinics? The prospective COVID-19 cohort study: protect-CoV
Abstract
Limited knowledge exists on the effectiveness of preventive preparedness plans for the care of outpatient cancer patients during epidemics or pandemics. To ensure adequate, timely and continuous clinical care for this highly vulnerable population, we propose the establishment of preventive standard safety protocols providing effective early phase identification of outbreaks at outpatient cancer facilities and communicating adapted standards of care. The prospective cohort study Protect-CoV conducted at the LMU Klinikum from mid-March to June 2020 investigated the effectiveness of a rapid, proactive and methodical response to protect patients and interrupt SARS-CoV-2 transmission chains during the first pandemic wave. The implemented measures reduced the risk of infection of individual cancer patients and ensured safe adjunctive infusion therapy in an outpatient setting during the early COVID-19 pandemic. In addition to the immediate implementation of standard hygiene procedures, our results underscore the importance of routine PCR testing for the identification of asymptomatic or pre-symptomatic COVID-19 cases and immediate tracing of positive cases and their contacts. While more prospective controlled studies are needed to confirm these results, our study illustrates the importance of including preventative testing and tracing measures in the standard risk reduction procedures at all out patient cancer centers.
Keywords: Cancer; Covid-19; Immunology; Outpatient; Pandemic; SARS-CoV-2.
© 2022. The Author(s).
Conflict of interest statement
AS received honoraria from Roche, Taiho Pharmaceutical and Servier and expenses for travel and accommodations from Roche, Merck KGaA, Amgen, Pfizer and Lilly Oncology. N.E. has received honoraria for participating in symposia and/or advisory boards for CSL Behring, Fresenius, Baxter, Havas Lynx Group, Janssen-Cliag, Nutricia and GHD and received funds from grants or contracts with the Techniker Health Insurance, the non-profit organization Eat what you need e.V. and Klarigo Publishers. Von B.B received honoraria from AMGEN, MSD Sharp & Dohme, Novartis, Roche, KITE/Gilead, Bristol-Myers Squibb, Astellas, Mologen and Miltenyi. H.B., A.G., S.K., have received grants from the Bavarian State Ministry for the Environment and Health, and from the Molecular Genetic Surveillance Network in Bavaria for the identification of SAR-CoV-2: Bay-VOC (variants of concern). V. H. has received honoraria for participating in symposia and advisory boards for Merck, Amgen, Roche, Sanofi, Boehringer Ingelheim, Celgene, Bayer, Pfizer, Sirtex Medical, SERVIER, MSD, Bristol-Myers Squibb, MSD Oncology, Novartis, Pierre Fabre. He has also received research funding from Merk, Amgen, Roche, Sanofi, Pfizer, Boehringer Ingelheim, Sirtex Medical, and Bayer and travel support from Merck, Roche, Sirtex Medical, Amgen, SERVIER, Shire, MSD and Bristol-Myers Squibb. None of these activities were related to the content of this article. T. Schinköthe is owner and Managing Director of CANKADO and owns stock in the company. All remaining authors declared no conflicts of interest.
Figures

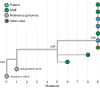
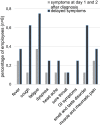
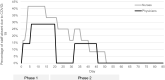
References
-
- Organization WH. COVID-19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. 2020. https://www.who.int/publications/m/item/covid-19-public-health-emergency.... Accessed 17 Oct 2021.
-
- Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, Chackathayil J, Cheng VW, Curley HM, Fittall MW, Freeman-Mills L, Gennatas S, Goel A, Hartley S, Hughes DJ, Kerr D, Lee AJ, Lee RJ, McGrath SE, Middleton CP, Murugaesu N, Newsom-Davis T, Okines AF, Olsson-Brown AC, Palles C, Pan Y, Pettengell R, Powles T, Protheroe EA, Purshouse K, Sharma-Oates A, Sivakumar S, Smith AJ, Starkey T, Turnbull CD, Várnai C, Yousaf N, Kerr R, Middleton G. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926. doi: 10.1016/s0140-6736(20)31173-9. - DOI - PMC - PubMed
-
- Angelis V, Tippu Z, Joshi K, Reis S, Gronthoud F, Fribbens C, Okines A, Stanway S, Cottier E, McGrath S, Watkins D, Noble J, Bhosle J, Gerlinger M, Hamid I, Soliman H, Nenclares P, Jones R, Harrington K, Gennatas S. Defining the true impact of coronavirus disease 2019 in the at-risk population of patients with cancer. Eur J Cancer. 2020;136:99–106. doi: 10.1016/j.ejca.2020.06.027. - DOI - PMC - PubMed
-
- Nichetti F, Bini M, Ambrosini M, Ottini A, Rametta A, Leporati R, Polastri D, Pircher C, Dotti K, Ferrari L, de Braud F. COVID-19 risk for patients undergoing anticancer treatment at the outpatient clinic of the National Cancer Institute of Milan: the COVINT study. ESMO Open. 2020 doi: 10.1136/esmoopen-2020-000883. - DOI - PMC - PubMed
-
- Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, Mehta RS, Warner ET, Sikavi DR, Lo CH, Kwon S, Song M, Mucci LA, Stampfer MJ, Willett WC, Eliassen AH, Hart JE, Chavarro JE, Rich-Edwards JW, Davies R, Capdevila J, Lee KA, Lochlainn MN, Varsavsky T, Sudre CH, Cardoso MJ, Wolf J, Spector TD, Ourselin S, Steves CJ, Chan AT. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475–e483. doi: 10.1016/s2468-2667(20)30164-x. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

