Propidium uptake and ATP release in A549 cells share similar transport mechanisms
- PMID: 35398020
- PMCID: PMC9117937
- DOI: 10.1016/j.bpj.2022.04.007
Propidium uptake and ATP release in A549 cells share similar transport mechanisms
Abstract
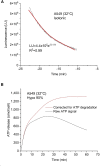
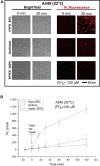
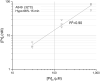
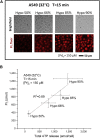
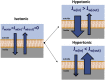
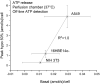
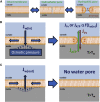
The lipid bilayer of eukaryotic cells' plasma membrane is almost impermeable to small ions and large polar molecules, but its miniscule basal permeability in intact cells is poorly characterized. This report describes the intrinsic membrane permeability of A549 cells toward the charged molecules propidium (Pr2+) and ATP4-. Under isotonic conditions, we detected with quantitative fluorescence microscopy, a continuous low-rate uptake of Pr (∼150 × 10-21 moles (zmol)/h/cell, [Pr]o = 150 μM, 32°C). It was stimulated transiently but strongly by 66% hypotonic cell swelling reaching an influx amplitude of ∼1500 (zmol/h)/cell. The progressive Pr uptake with increasing [Pr]o (30, 150, and 750 μM) suggested a permeation mechanism by simple diffusion. We quantified separately ATP release with custom wide-field-of-view chemiluminescence imaging. The strong proportionality between ATP efflux and Pr2+ influx during hypotonic challenge, and the absence of stimulation of transmembrane transport following 300% hypertonic shock, indicated that ATP and Pr travel the same conductive pathway. The fluorescence images revealed a homogeneously distributed intracellular uptake of Pr not consistent with high-conductance channels expressed at low density on the plasma membrane. We hypothesized that the pathway consists of transiently formed water pores evenly spread across the plasma membrane. The abolition of cell swelling-induced Pr uptake with 500 μM gadolinium, a known modulator of membrane fluidity, supported the involvement of water pores whose formation depends on the membrane fluidity. Our study suggests an alternative model of a direct permeation of ATP (and other molecules) through the phospholipid bilayer, which may have important physiological implications.
Keywords: ATP release; imaging; osmotic shock; propidium uptake.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Tan J.J., Boudreault F., Grygorczyk R. Mechanosensitive ATP release through transient membrane rupture in alveolar epithelial cells. FASEB J. 2020;34:1. doi: 10.1096/fasebj.2020.34.s1.06107. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

