Impact of the SARS-CoV-2 pandemic on vaccine-preventable disease campaigns
- PMID: 35398300
- PMCID: PMC8985404
- DOI: 10.1016/j.ijid.2022.04.005
Impact of the SARS-CoV-2 pandemic on vaccine-preventable disease campaigns
Abstract
Background: The COVID-19 pandemic has contributed to the widespread disruption of immunization services, including the postponement of mass vaccination campaigns.
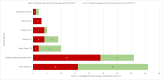
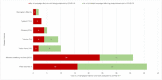
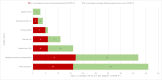
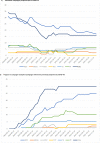
Methods: In May 2020, the World Health Organization and partners started monitoring COVID-19-related disruptions to mass vaccination campaigns against cholera, measles, meningitis A, polio, tetanus-diphtheria, typhoid, and yellow fever through the Immunization Repository Campaign Delay Tracker. The authors reviewed the number and target population of reported preventive and outbreak response vaccination campaigns scheduled, postponed, canceled, and reinstated at 4 time points: May 2020, December 2020, May 2021, and December 2021.
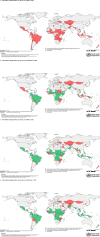
Findings: Mass vaccination campaigns across all vaccines were disrupted heavily by COVID-19. In May 2020, 105 of 183 (57%) campaigns were postponed or canceled in 57 countries because of COVID-19, with an estimated 796 million postponed or missed vaccine doses. Campaign resumption was observed beginning in July 2020. In December 2021, 77 of 472 (16%) campaigns in 54 countries, mainly in the African Region, were still postponed or canceled because of COVID-19, with about 382 million postponed or missed vaccine doses.
Interpretation: There is likely a high risk of vaccine-preventable disease outbreaks across all regions because of an increased number of susceptible persons resulting from the large-scale mass vaccination campaign postponement caused by COVID-19.
Keywords: COVID; SARS-CoV-2 pandemic; campaign; global; vaccination; vaccine-preventable diseases.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no competing interests.
Figures
References
-
- Causey K, Fullman N, Sorensen RJD, Galles NC, Zheng P, Aravkin A, et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: a modeling study. Lancet 2021. 2021;398:522–534. https://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(21)01337-4.pdf - PMC - PubMed
-
- EYE newsletter August 2020. https://mailchi.mp/c01237c9ed5c/eye-newsletter-august-2020?e=e98b6f0e1c (accessed March 20, 2022).
-
- Global Polio Eradication Initiative: GPEI (2020). Polio Eradication in the Context of the COVID-19 Pandemic: Summary of urgent country and regional recommendations from the Polio Oversight Board meeting of March 24, 2020. https://polioeradication.org/wp-content/uploads/2020/04/POB-meeting-summ... (accessed March 20, 2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

