Androgen Regulation of Corticotropin Releasing Factor Receptor 1 in the Mouse Brain
- PMID: 35398506
- PMCID: PMC9489527
- DOI: 10.1016/j.neuroscience.2022.04.005
Androgen Regulation of Corticotropin Releasing Factor Receptor 1 in the Mouse Brain
Abstract
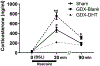
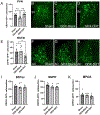
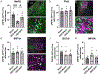
Stress-related mood disorders like anxiety and depression are more prevalent in women than men and are often associated with hypothalamic-pituitary-adrenal (HPA) axis dysregulation. Androgen actions through androgen receptors (ARs) decrease HPA axis responses and stress-associated behaviors. Corticotropin releasing factor (CRF) and its binding to CRF receptor 1 (CRFR1) is also critical for regulation of the HPA axis, anxiety, and depression. We first determined CRFR1/AR co-localization patterns in male and female CRFR1-GFP mice. High co-localization was found within the paraventricular nucleus (PVN), dorsolateral and anteroventral subdivisions of the bed nucleus of the stria terminalis (BSTdl and BSTav), medial preoptic area (MPOA), and posterodorsal medial amygdala (MePD). We next determined whether the non-aromatizable androgen dihydrotestosterone (DHT) regulates CRFR1 expression and stress-induced activation of CRFR1-expressing cells. In the PVN, CRFR1-GFP cell number decreased following gonadectomy (GDX), but DHT treatment reversed this effect. GDX-DHT treated mice also had a decreased CRFR1-GFP cell number within the BSTdl compared to gonad intact and GDX-untreated groups. Following restraint stress GDX-blank mice showed fewer c-Fos/CRFR1 co-localized neurons in the MePD compared to gonad intact and GDX-DHT groups indicating decreased stress-induced activation of CRFR1 neurons following GDX. Higher plasma corticosterone (CORT) was found in GDX males compared to GDX-DHT and sham males following restraint stress, with a negative correlation between PVN CRFR1+ neurons and corticosterone levels 30- and 90-min following restraint. Together these findings show androgens can directly alter CRFR1 levels in the brain which may have implications for sex differences in regulation of the HPA axis and stress-related behaviors.
Keywords: Stress; androgen; androgen receptor; corticotropin releasing factor; hypothalamic–pituitary–adrenal axis; sex difference.
Copyright © 2022 IBRO. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
None declared.
Figures




Similar articles
-
Sex differences in androgen receptor, estrogen receptor alpha, and c-Fos co-expression with corticotropin releasing factor expressing neurons in restrained adult mice.Horm Behav. 2023 Nov;156:105448. doi: 10.1016/j.yhbeh.2023.105448. Epub 2023 Oct 27. Horm Behav. 2023. PMID: 38344954 Free PMC article.
-
A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus.Neuroscience. 2019 Jun 15;409:195-203. doi: 10.1016/j.neuroscience.2019.04.045. Epub 2019 May 2. Neuroscience. 2019. PMID: 31055007 Free PMC article.
-
Androgen inhibits the increases in hypothalamic corticotropin-releasing hormone (CRH) and CRH-immunoreactivity following gonadectomy.Neuroendocrinology. 1994 Mar;59(3):228-34. doi: 10.1159/000126663. Neuroendocrinology. 1994. PMID: 8159272
-
Sex differences in circadian timing systems: implications for disease.Front Neuroendocrinol. 2014 Jan;35(1):111-39. doi: 10.1016/j.yfrne.2013.11.003. Epub 2013 Nov 25. Front Neuroendocrinol. 2014. PMID: 24287074 Free PMC article. Review.
-
Brain mechanisms of HPA axis regulation: neurocircuitry and feedback in context Richard Kvetnansky lecture.Stress. 2020 Nov;23(6):617-632. doi: 10.1080/10253890.2020.1859475. Epub 2020 Dec 21. Stress. 2020. PMID: 33345670 Free PMC article. Review.
Cited by
-
Androgen regulation of behavioral stress responses and the hypothalamic-pituitary-adrenal axis.Horm Behav. 2024 Jun;162:105528. doi: 10.1016/j.yhbeh.2024.105528. Epub 2024 Mar 18. Horm Behav. 2024. PMID: 38503191 Free PMC article. Review.
-
A subpopulation of oxytocin neurons initiate expression of CRF receptor 1 (CRFR1) in females post parturition.Psychoneuroendocrinology. 2022 Nov;145:105918. doi: 10.1016/j.psyneuen.2022.105918. Epub 2022 Sep 7. Psychoneuroendocrinology. 2022. PMID: 36116320 Free PMC article.
-
Sex differences in androgen receptor, estrogen receptor alpha, and c-Fos co-expression with corticotropin releasing factor expressing neurons in restrained adult mice.Horm Behav. 2023 Nov;156:105448. doi: 10.1016/j.yhbeh.2023.105448. Epub 2023 Oct 27. Horm Behav. 2023. PMID: 38344954 Free PMC article.
-
Dominance status modulates activity in medial amygdala cells with projections to the bed nucleus of the stria terminalis.Behav Brain Res. 2023 Sep 13;453:114628. doi: 10.1016/j.bbr.2023.114628. Epub 2023 Aug 12. Behav Brain Res. 2023. PMID: 37579818 Free PMC article.
-
Sex differences in the relationship between depression and Alzheimer's disease-mechanisms, genetics, and therapeutic opportunities.Front Aging Neurosci. 2024 Jun 5;16:1301854. doi: 10.3389/fnagi.2024.1301854. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38903903 Free PMC article. Review.
References
-
- Bingham B, Myung C, Innala L, Gray M, Anonuevo A, Viau V. Androgen receptors in the posterior bed nucleus of the stria terminalis increase neuropeptide expression and the stress-induced activation of the paraventricular nucleus of the hypothalamus. Neuropsychopharmacology. 2011. Jun;36(7):1433–43. doi: 10.1038/npp.2011.27. Epub 2011 Mar 16. - DOI - PMC - PubMed
-
- Chen CV, Brummet JL, Jordan CL, Breedlove SM. Down, But Not Out: Partial Elimination of Androgen Receptors in the Male Mouse Brain Does Not Affect Androgenic Regulation of Anxiety or HPA Activity. Endocrinology. 2016. Feb;157(2):764–73. doi: 10.1210/en.2015-1417. Epub 2015 Nov 12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

