The CEL-HYB1 Hybrid Allele Promotes Digestive Enzyme Misfolding and Pancreatitis in Mice
- PMID: 35398595
- PMCID: PMC9117557
- DOI: 10.1016/j.jcmgh.2022.03.013
The CEL-HYB1 Hybrid Allele Promotes Digestive Enzyme Misfolding and Pancreatitis in Mice
Abstract
Background & aims: A hybrid allele that originated from homologous recombination between CEL and its pseudogene (CELP), CEL-HYB1 increases the risk of chronic pancreatitis (CP). Although suggested to cause digestive enzyme misfolding, definitive in vivo evidence for this postulate has been lacking.
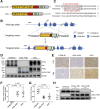
Methods: CRISPR-Cas9 was used to generate humanized mice harboring the CEL-HYB1 allele on a C57BL/6J background. Humanized CEL mice and C57BL/6J mice were used as controls. Pancreata were collected and analyzed by histology, immunohistochemistry, immunoblotting, and transcriptomics. Isolated pancreatic acini were cultured in vitro to measure the secretion and aggregation of CEL-HYB1 protein. Mice were given caerulein injections to induce acute pancreatitis (AP) and CP.
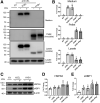
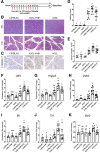
Results: Pancreata from mice expressing CEL-HYB1 developed pathological features characteristic of focal pancreatitis that included acinar atrophy and vacuolization, inflammatory infiltrates, and fibrosis in a time-dependent manner. CEL-HYB1 expression in pancreatic acini led to decreased secretion and increased intracellular aggregation and triggered endoplasmic reticulum stress compared with CEL. The autophagy levels of pancreata from mice expressing CEL-HYB1 changed at different developmental stages; some aged CEL-HYB1 mice exhibited an accumulation of large autophagic vesicles and impaired autophagy in acinar cells. Administration of caerulein increased the severity of AP/CP in mice expressing CEL-HYB1 compared with control mice, accompanied by higher levels of endoplasmic reticulum stress.
Conclusions: Expression of a humanized form of CEL-HYB1 in mice promotes endoplasmic reticulum stress and pancreatitis through a misfolding-dependent pathway. Impaired autophagy appears to be involved in the pancreatic injury in aged CEL-HYB1 mice. These mice have the potential to be used as a model to identify therapeutic targets for CP.
Keywords: Carboxyl Ester Lipase; Chronic Pancreatitis; Genetic Variants; Protein Misfolding.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Figures








References
-
- Gardner T.B., Adler D.G., Forsmark C.E., Sauer B.G., Taylor J.R., Whitcomb D.C. ACG clinical guideline: chronic pancreatitis. Am J Gastroenterol. 2020;115:322–339. - PubMed
-
- Chiari H. Über die Selbstverdauung des menschlichen Pankreas. Z Heilkunde. 1896;17:69–96.
-
- Whitcomb D.C., Gorry M.C., Preston R.A., Furey W., Sossenheimer M.J., Ulrich C.D., Martin S.P., Gates L.K., Jr., Amann S.T., Toskes P.P., Liddle R., McGrath K., Uomo G., Post J.C., Ehrlich G.D. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. - PubMed
-
- Le Maréchal C., Masson E., Chen J.M., Morel F., Ruszniewski P., Levy P., Férec C. Hereditary pancreatitis caused by triplication of the trypsinogen locus. Nat Genet. 2006;38:1372–1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

