"The emerging role of capivasertib in breast cancer"
- PMID: 35398754
- PMCID: PMC9011110
- DOI: 10.1016/j.breast.2022.03.018
"The emerging role of capivasertib in breast cancer"
Abstract
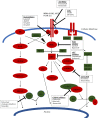
Over 50% of breast tumors harbor alterations in one or more genes of the phosphatidylinositol 3-kinase (PI3K) pathway including PIK3CA mutations (31%), PTEN loss (34%), PTEN mutations (5%) and AKT1 mutations (3%). While PI3K and mTOR inhibitors are already approved in advanced breast cancer, AKT inhibitors have been recently developed as a new therapeutic approach. Capivasertib (AZD5363) is a novel, selective ATP-competitive pan-AKT kinase inhibitor that exerts similar activity against the three AKT isoforms, AKT1, AKT2, and AKT3. Preclinical studies demonstrated efficacy of capivasertib in breast cancer cell lines as a single agent or in combination with anti-HER2 agents and endocrine treatment, especially in tumors with PIK3CA or MTOR alterations. Phase I/II studies demonstrated greater efficacy when capivasertib was co-administered with paclitaxel, fulvestrant in hormone receptor (HR)-positive, HER2-negative breast cancer or olaparib. The recommended phase II dose of capivasertib as monotherapy was 480 mg bid on a 4-days-on, 3-days-off dosing schedule. Toxicity profile proved to be manageable with hyperglycemia (20-24%), diarrhea (14-17%) and maculopapular rash (11-16%) being the most common grade ≥3 adverse events. Ongoing Phase III trials of capivasertib in combination with fulvestrant (CAPItello-291), CDK4/6 inhibitor palbociclib (CAPItello-292) and paclitaxel (CAPItello- 290) will better clarify the therapeutic role of capivasertib in breast cancer.
Keywords: Capivasertib”; “AKT inhibitor”; “Breast cancer”; “PI3K/AKT/mTOR pathway”.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- FDA approves alpelisib for metastatic breast cancer | FDA n.d. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro... (accessed January 30, 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous