Boosting of serum neutralizing activity against the Omicron variant among recovered COVID-19 patients by BNT162b2 and CoronaVac vaccines
- PMID: 35398786
- PMCID: PMC8989491
- DOI: 10.1016/j.ebiom.2022.103986
Boosting of serum neutralizing activity against the Omicron variant among recovered COVID-19 patients by BNT162b2 and CoronaVac vaccines
Abstract
Background: SARS-CoV-2 Omicron variant evades immunity from past infection or vaccination and is associated with a greater risk of reinfection among recovered COVID-19 patients. We assessed the serum neutralizing antibody (NAb) activity against Omicron variant (Omicron NAb) among recovered COVID-19 patients with or without vaccination.
Methods: In this prospective cohort study with 135 recovered COVID-19 patients, we determined the serum NAb titers against ancestral virus or variants using a live virus NAb assay. We used the receiver operating characteristic analysis to determine the optimal cutoff for a commercially-available surrogate NAb assay.
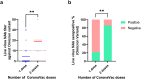
Findings: Among recovered COVID-19 patients, the serum live virus geometric mean Omicron NAb titer was statistically significantly higher among BNT162b2 recipients compared to non-vaccinated individuals (85.4 vs 5.6,P < 0.0001). The Omicron seropositive rates in live virus NAb test (NAb titer ≥10) were statistically significantly higher among BNT162b2 (90.6% [29/32];P < 0.0001) or CoronaVac (36.7% [11/30]; P = 0.0115) recipients when compared with non-vaccinated individuals (12.3% [9/73]). Subgroup analysis of CoronaVac recipients showed that the Omicron seropositive rates were higher among individuals with two doses than those with one dose (85.7% vs 21.7%; P = 0.0045). For the surrogate NAb assay, a cutoff of 109.1 AU/ml, which is 7.3-fold higher than the manufacturer's recommended cutoff, could achieve a sensitivity and specificity of 89.5% and 89.8%, respectively, in detecting Omicron NAb.
Interpretation: Among individuals with prior COVID-19, one dose of BNT162b2 or two doses of CoronaVac could induce detectable serum Omicron NAb. Our result would be particularly important for guiding vaccine policies in countries with COVID-19 vaccine shortage.
Funding: Health and Medical Research Fund, Richard and Carol Yu, Michael Tong (see acknowledgments for full list).
Keywords: Beta variant; COVID-19; Delta variant; Inactivated vaccine; Neutralizing antibody; Omicron variant; SARS-CoV-2; Spike protein receptor binding domain; Surrogate neutralizing antibody test; mRNA vaccine.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests KYY and KKWT report collaboration with SinoVac and Sinopharm. IFNH report collaboration with Sinovac, Sinopharm, Pfizer-BNT-Fosun, and MSD. Other authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

