Stevens-Johnson syndrome precipitated by Moderna Inc. COVID-19 vaccine: a case-based review of literature comparing vaccine and drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis
- PMID: 35398905
- PMCID: PMC9111554
- DOI: 10.1111/ijd.16222
Stevens-Johnson syndrome precipitated by Moderna Inc. COVID-19 vaccine: a case-based review of literature comparing vaccine and drug-induced Stevens-Johnson syndrome/toxic epidermal necrolysis
Abstract
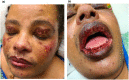
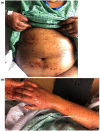
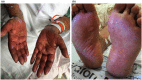
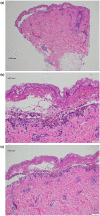
The Moderna COVID-19 vaccination was approved for use in the United States in December of 20201 and since that time massive public health efforts have been made to vaccinate patients against the COVID-19 infection. Adverse reactions from the vaccination are well-reported and include both local skin reactions, such as pain, swelling, and erythema at the injection site, as well as systemic reactions including fever, malaise, headache, muscle aches, drowsiness, nausea, and vomiting. While severe serious cutaneous adverse reactions, such as Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN), remain rare; two cases of SJS/TEN related to COVID-19 vaccination have been reported. We herein review the two previously reported cases of SJS/TEN and report the first case of SJS precipitated by the Moderna Inc., MRNA 1273 COVID-19 vaccination in the United States. Although we review potential adverse reactions to vaccination, the benefits of COVID-19 vaccination outweigh the risks based on current data. Cases should be reported to the Vaccine Adverse Event Reporting System (https://vaers.hhs.gov/) to help public health officials recognize and track these severe but rare adverse events.
© 2022 the International Society of Dermatology.
Conflict of interest statement
None declared.
Figures
Similar articles
-
An extremely rare mucocutaneous adverse reaction following COVID-19 vaccination: Toxic epidermal necrolysis.Dermatol Ther. 2022 May;35(5):e15416. doi: 10.1111/dth.15416. Epub 2022 Mar 9. Dermatol Ther. 2022. PMID: 35238119 Free PMC article.
-
Background rates for severe cutaneous reactions in the US: Contextual support for safety assessment of COVID-19 vaccines and novel biologics.Vaccine. 2023 Nov 13;41(47):6922-6929. doi: 10.1016/j.vaccine.2023.10.025. Epub 2023 Oct 25. Vaccine. 2023. PMID: 37891051 Review.
-
Toxic epidermal necrolysis and Stevens-Johnson syndrome after COVID-19 infection and vaccination.Australas J Dermatol. 2023 Feb;64(1):e1-e10. doi: 10.1111/ajd.13958. Epub 2022 Dec 9. Australas J Dermatol. 2023. PMID: 36484649 Free PMC article. Review.
-
Erythema multiforme, Stevens Johnson syndrome, and toxic epidermal necrolysis reported after vaccination, 1999-2017.Vaccine. 2020 Feb 11;38(7):1746-1752. doi: 10.1016/j.vaccine.2019.12.028. Epub 2019 Dec 20. Vaccine. 2020. PMID: 31870573 Free PMC article.
-
The seven-fold rise in incidence of Stevens-Johnson syndrome & toxic epidermal necrolysis: Associations with COVID-19 and the vaccine.Burns. 2024 Feb;50(1):87-92. doi: 10.1016/j.burns.2023.06.016. Epub 2023 Jun 28. Burns. 2024. PMID: 37730480
Cited by
-
A comprehensive review of oral microenvironment changes and orofacial adverse reactions after COVID-19 vaccination: The good, the bad, and the ugly.Health Sci Rep. 2024 Mar 13;7(3):e1967. doi: 10.1002/hsr2.1967. eCollection 2024 Mar. Health Sci Rep. 2024. PMID: 38482134 Free PMC article.
-
Toxic Epidermal Necrolysis after COVID-19 mRNA-1237 Vaccination.Case Reports Immunol. 2023 Nov 14;2023:8855665. doi: 10.1155/2023/8855665. eCollection 2023. Case Reports Immunol. 2023. PMID: 38021339 Free PMC article.
-
Pathophysiology of oral lesions subsequent to SARS-CoV-2 vaccination: A systematic review.J Oral Maxillofac Pathol. 2024 Jul-Sep;28(3):443-454. doi: 10.4103/jomfp.jomfp_511_23. Epub 2024 Oct 15. J Oral Maxillofac Pathol. 2024. PMID: 39670118 Free PMC article. Review.
-
Toxic Epidermal Necrolysis After COVID-19 Vaccination: A Case Report and Review of Literature.J Acute Med. 2024 Jun 1;14(2):94-97. doi: 10.6705/j.jacme.202406_14(2).0005. J Acute Med. 2024. PMID: 38855049 Free PMC article.
-
Toxic epidermal necrolysis after first dose of Pfizer-BioNTech (BNT162b2) vaccination with pharmacogenomic testing.Pediatr Dermatol. 2022 Jul;39(4):601-605. doi: 10.1111/pde.15074. Pediatr Dermatol. 2022. PMID: 36000937 Free PMC article.
References
-
- Fact sheet for recipients and caregivers emergency use authorization (eua) of the moderna covid‐19 vaccine to prevent coronavirus disease 2019 (covid‐19) in individuals 18 years of age and older . https://www.fda.gov/media/144638/download#page=2a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

