Human papilloma virus integration sites and genomic signatures in head and neck squamous cell carcinoma
- PMID: 35398964
- PMCID: PMC9394244
- DOI: 10.1002/1878-0261.13219
Human papilloma virus integration sites and genomic signatures in head and neck squamous cell carcinoma
Abstract
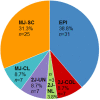
A prevalence of around 26% of human papillomavirus (HPV) in head and neck squamous cell carcinoma (HNSCC) has been previously reported. HPV induced oncogenesis mainly involving E6 and E7 viral oncoproteins. In some cases, HPV viral DNA has been detected to integrate with the host genome and possibly contributes to carcinogenesis by affecting the gene expression. We retrospectively assessed HPV integration sites and signatures in 80 HPV positive patients with HNSCC, by using a double capture-HPV method followed by next-generation Sequencing. We detected HPV16 in 90% of the analyzed cohort and confirmed five previously described mechanistic signatures of HPV integration [episomal (EPI), integrated in a truncated form revealing two HPV-chromosomal junctions colinear (2J-COL) or nonlinear (2J-NL), multiple hybrid junctions clustering in a single chromosomal region (MJ-CL) or scattered over different chromosomal regions (MJ-SC) of the human genome]. Our results suggested that HPV remained episomal in 38.8% of the cases or was integrated/mixed in the remaining 61.2% of patients with HNSCC. We showed a lack of association of HPV genomic signatures to tumour and patient characteristics, as well as patient survival. Similar to other HPV associated cancers, low HPV copy number was associated with worse prognosis. We identified 267 HPV-human junctions scattered on most chromosomes. Remarkably, we observed four recurrent integration regions: PDL1/PDL2/PLGRKT (8.2%), MYC/PVT1 (6.1%), MACROD2 (4.1%) and KLF5/KLF12 regions (4.1%). We detected the overexpression of PDL1 and MYC upon integration by gene expression analysis. In conclusion, we identified recurrent targeting of several cancer genes such as PDL1 and MYC upon HPV integration, suggesting a role of altered gene expression by HPV integration during HNSCC carcinogenesis.
Keywords: MYC; PDL1; HPV copy number; HPV integration; carcinogenesis; head and neck squamous cell carcinoma.
© 2022 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
All other authors report no conflict of interest.
Figures



References
-
- Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M‐J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head‐and‐neck squamous cell carcinoma (KEYNOTE‐040): a randomised, open‐label, phase 3 study. Lancet. 2019;393(10167):156–67. 10.1016/S0140-6736(18)31999-8 - DOI - PubMed
-
- Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet. 2019;394(10212):1915–28. 10.1016/S0140-6736(19)32591-7 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

