Outcome of aggressive B-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT
- PMID: 35399106
- PMCID: PMC8995369
- DOI: 10.1038/s41392-022-00924-0
Outcome of aggressive B-cell lymphoma with TP53 alterations administered with CAR T-cell cocktail alone or in combination with ASCT
Abstract
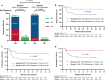
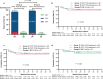
TP53 gene alteration confers inferior prognosis in refractory/relapse aggressive B-cell non-Hodgkin lymphoma (r/r B-NHL). From September 2016 to September 2020, 257 r/r B-NHL patients were assessed for eligibility for two trials in our center, assessing anti-CD19 and anti-CD22 chimeric antigen receptor (CAR19/22) T-cell cocktail treatment alone or in combination with autologous stem cell transplantation (ASCT). TP53 alterations were screened in 123 enrolled patients and confirmed in 60. CAR19/22 T-cell administration resulted in best objective (ORR) and complete (CRR) response rate of 87.1% and 45.2% in patients with TP53 alterations, respectively. Following a median follow-up of 16.7 months, median progression-free survival (PFS) was 14.8 months, and 24-month overall survival (OS) was estimated at 56.3%. Comparable ORR, PFS, and OS were determined in individuals with or without TP53 alterations, and in individuals at different risk levels based on functional stratification of TP53 alterations. CAR19/22 T-cell treatment in combination with ASCT resulted in higher ORR, CRR, PFS, and OS, but reduced occurrence of severe CRS in this patient population, even in individuals showing stable or progressive disease before transplantation. The best ORR and CRR in patients with TP53 alterations were 92.9% and 82.1%, respectively. Following a median follow-up of 21.2 months, 24-month PFS and OS rates in patients with TP53 alterations were estimated at 77.5% and 89.3%, respectively. In multivariable analysis, this combination strategy predicted improved OS. In conclusion, CAR19/22 T-cell therapy is efficacious in r/r aggressive B-NHL with TP53 alterations. Combining CAR-T cell administration with ASCT further improves long-term outcome of these patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Jacobson C, et al. Long-term survival and gradual recovery of B cells in patients with refractory large B cell lymphoma treated with axicabtagene ciloleucel (Axi-Cel) Blood. 2020;136:40–42.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

