Arterial Baroreflex Inhibits Muscle Metaboreflex Induced Increases in Effective Arterial Elastance: Implications for Ventricular-Vascular Coupling
- PMID: 35399256
- PMCID: PMC8990766
- DOI: 10.3389/fphys.2022.841076
Arterial Baroreflex Inhibits Muscle Metaboreflex Induced Increases in Effective Arterial Elastance: Implications for Ventricular-Vascular Coupling
Abstract
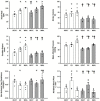
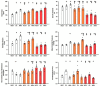
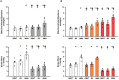
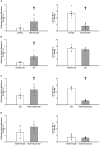
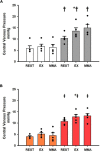
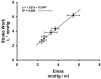
The ventricular-vascular relationship assesses the efficacy of energy transferred from the left ventricle to the systemic circulation and is quantified as the ratio of effective arterial elastance to maximal left ventricular elastance. This relationship is maintained during exercise via reflex increases in cardiovascular performance raising both arterial and ventricular elastance in parallel. These changes are, in part, due to reflexes engendered by activation of metabosensitive skeletal muscle afferents-termed the muscle metaboreflex. However, in heart failure, ventricular-vascular uncoupling is apparent and muscle metaboreflex activation worsens this relationship through enhanced systemic vasoconstriction markedly increasing effective arterial elastance which is unaccompanied by substantial increases in ventricular function. This enhanced arterial vasoconstriction is, in part, due to significant reductions in cardiac performance induced by heart failure causing over-stimulation of the metaboreflex due to under perfusion of active skeletal muscle, but also as a result of reduced baroreflex buffering of the muscle metaboreflex-induced peripheral sympatho-activation. To what extent the arterial baroreflex modifies the metaboreflex-induced changes in effective arterial elastance is unknown. We investigated in chronically instrumented conscious canines if removal of baroreflex input via sino-aortic baroreceptor denervation (SAD) would significantly enhance effective arterial elastance in normal animals and whether this would be amplified after induction of heart failure. We observed that effective arterial elastance (Ea), was significantly increased during muscle metaboreflex activation after SAD (0.4 ± 0.1 mmHg/mL to 1.4 ± 0.3 mmHg/mL). In heart failure, metaboreflex activation caused exaggerated increases in Ea and in this setting, SAD significantly increased the rise in Ea elicited by muscle metaboreflex activation (1.3 ± 0.3 mmHg/mL to 2.3 ± 0.3 mmHg/mL). Thus, we conclude that the arterial baroreflex does buffer muscle metaboreflex induced increases in Ea and this buffering likely has effects on the ventricular-vascular coupling.
Keywords: arterial baroreflex; effective arterial elastance (Ea); muscle metaboreflex activation; neural control of cardiovascular system; ventricular vascular coupling.
Copyright © 2022 Mannozzi, Kim, Sala-Mercado, Al-Hassan, Lessanework, Alvarez, Massoud, Bhatti, Aoun and O’Leary.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

