A Novel Organ-Specific Approach to Selectively Target Sensory Afferents Innervating the Aortic Arch
- PMID: 35399269
- PMCID: PMC8987286
- DOI: 10.3389/fphys.2022.841078
A Novel Organ-Specific Approach to Selectively Target Sensory Afferents Innervating the Aortic Arch
Abstract
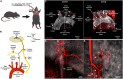
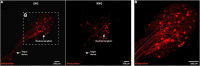
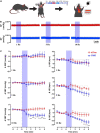
The brain maintains cardiovascular homeostasis, in part, via the arterial baroreflex which senses changes in blood pressure (BP) at the level of the aortic arch. Sensory afferents innervating the aortic arch employ baroreceptors to convert stretch exerted on the arterial wall into action potentials carried by the vagus nerve to second order neurons residing within the nucleus of the solitary tract (NTS). Although the baroreflex was described more than 80 years ago, the specific molecular, structural, and functional phenotype of the baroreceptors remain uncharacterized. This is due to the lack of tools that provide the genetic and target organ specificity that is required to selectively characterize baroreceptor afferents. Here, we use a novel approach to selectively target baroreceptors. Male mice on a C57BL/6J background were anesthetized with isoflurane, intubated, and artificially ventilated. Following sternotomy, the aortic arch was exposed, and a retrograde adeno-associated virus was applied to the aortic arch to direct the expression of channelrhoropsin-2 (ChR2) and/or tdTomato (tdTom) to sensory afferents presumably functioning as baroreceptors. Consistent with the structural characteristics of arterial baroreceptors, robust tdTom expression was observed in nerve endings surrounding the aortic arch, within the fibers of the aortic depressor and vagus nerves, cell bodies of the nodose ganglia (NDG), and neural projections to the caudal NTS (cNTS). Additionally, the tdTom labeled cell bodies within the NDG also expressed mRNAs coding for the mechanically gated ion channels, PIEZO-1 and PIEZO-2. In vitro electrophysiology revealed that pulses of blue light evoked excitatory post-synaptic currents in a subset of neurons within the cNTS, suggesting a functional connection between the labeled aortic arch sensory afferents and second order neurons. Finally, the in vivo optogenetic stimulation of the cell bodies of the baroreceptor expressing afferents in the NDG produced robust depressor responses. Together, these results establish a novel approach for selectively targeting sensory neurons innervating the aortic arch. This approach may be used to investigate arterial baroreceptors structurally and functionally, and to assess their role in the etiology or reversal of cardiovascular disease.
Keywords: aortic arch; baroreceptors; baroreflex; blood pressure; nodose ganglia; sensory neurons; vagal afferents.
Copyright © 2022 Elsaafien, Harden, Johnson, Kimball, Sheng, Smith, Scott, Frazier, de Kloet and Krause.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Andresen M. C., Paton J. F. R. (2011). ““The Nucleus of the Solitary Tract: Processing Information from Viscerosensory Afferents,”,” in Central regulation of autonomic functions, eds Llewellyn-Smith I. J., Verberne A. J. M. (Oxford: Oxford University Press; ), 23–46. 10.1093/acprof:oso/9780195306637.003.0002 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

