C9orf72-Derived Proline:Arginine Poly-Dipeptides Modulate Cytoskeleton and Mechanical Stress Response
- PMID: 35399536
- PMCID: PMC8983821
- DOI: 10.3389/fcell.2022.750829
C9orf72-Derived Proline:Arginine Poly-Dipeptides Modulate Cytoskeleton and Mechanical Stress Response
Abstract
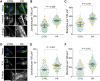
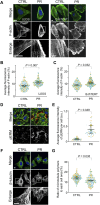
Proline:arginine (PR) poly-dipeptides from the GGGGCC repeat expansion in C9orf72 have cytotoxicity and bind intermediate filaments (IFs). However, it remains unknown how PR poly-dipeptides affect cytoskeletal organization and focal adhesion (FA) formation. Here, we show that changes to the cytoskeleton and FA by PR poly-dipeptides result in the alteration of cell stiffness and mechanical stress response. PR poly-dipeptides increased the junctions and branches of the IF network and increased cell stiffness. They also changed the distribution of actin filaments and increased the size of FA and intracellular calcium concentration. PR poly-dipeptides or an inhibitor of IF organization prevented cell detachment. Furthermore, PR poly-dipeptides induced upregulation of mechanical stress response factors and led to a maladaptive response to cyclic stretch. These results suggest that the effects of PR poly-dipeptides on mechanical properties and mechanical stress response may serve as a pathogenesis of C9orf72-related neurodegeneration.
Keywords: C9orf72; Focal Adhesion (FA); PR poly-dipeptides; actin; cytoskeleton.
Copyright © 2022 Shiota, Nagata, Kikuchi, Nanaura, Matsubayashi, Nakanishi, Kobashigawa, Isozumi, Kiriyama, Nagayama, Sugie, Yamashiro and Mori.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Proline-arginine poly-dipeptide encoded by the C9orf72 repeat expansion inhibits adenosine deaminase acting on RNA.J Neurochem. 2021 Aug;158(3):753-765. doi: 10.1111/jnc.15445. Epub 2021 Jun 24. J Neurochem. 2021. PMID: 34081786
-
Poly-dipeptides produced from C9orf72 hexanucleotide repeats cause selective motor neuron hyperexcitability in ALS.Proc Natl Acad Sci U S A. 2022 Mar 15;119(11):e2113813119. doi: 10.1073/pnas.2113813119. Epub 2022 Mar 8. Proc Natl Acad Sci U S A. 2022. PMID: 35259014 Free PMC article.
-
Nucleolar stress and impaired stress granule formation contribute to C9orf72 RAN translation-induced cytotoxicity.Hum Mol Genet. 2015 May 1;24(9):2426-41. doi: 10.1093/hmg/ddv005. Epub 2015 Jan 9. Hum Mol Genet. 2015. PMID: 25575510
-
Order controls disordered droplets: structure-function relationships in C9ORF72-derived poly(PR).Am J Physiol Cell Physiol. 2022 Feb 1;322(2):C197-C204. doi: 10.1152/ajpcell.00372.2021. Epub 2021 Dec 15. Am J Physiol Cell Physiol. 2022. PMID: 34910602 Review.
-
The Role of Dipeptide Repeats in C9ORF72-Related ALS-FTD.Front Mol Neurosci. 2017 Feb 13;10:35. doi: 10.3389/fnmol.2017.00035. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28243191 Free PMC article. Review.
Cited by
-
Interaction of the C9orf72-Amyotrophic Lateral Sclerosis-Related Proline-Arginine Dipeptide Repeat Protein with the RNA-Binding Protein NOVA1 Causes Decreased Expression of UNC13A Due to Enhanced Inclusion of Cryptic Exons, Which Is Reversed by Betulin Treatment.Cells. 2023 Oct 18;12(20):2476. doi: 10.3390/cells12202476. Cells. 2023. PMID: 37887320 Free PMC article.
-
Pectolinarigenin Improves Oxidative Stress and Apoptosis in Mouse NSC-34 Motor Neuron Cell Lines Induced by C9-ALS-Associated Proline-Arginine Dipeptide Repeat Proteins by Enhancing Mitochondrial Fusion Mediated via the SIRT3/OPA1 Axis.Antioxidants (Basel). 2023 Nov 16;12(11):2008. doi: 10.3390/antiox12112008. Antioxidants (Basel). 2023. PMID: 38001861 Free PMC article.
-
Multiple roles for the cytoskeleton in ALS.Exp Neurol. 2022 Sep;355:114143. doi: 10.1016/j.expneurol.2022.114143. Epub 2022 Jun 14. Exp Neurol. 2022. PMID: 35714755 Free PMC article. Review.
-
Biochemical Pathways of Cellular Mechanosensing/Mechanotransduction and Their Role in Neurodegenerative Diseases Pathogenesis.Cells. 2022 Oct 1;11(19):3093. doi: 10.3390/cells11193093. Cells. 2022. PMID: 36231055 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

