Clinically useful tumor fluorescence greater than 24 hours after 5-aminolevulinic acid administration
- PMID: 35399905
- PMCID: PMC8986640
- DOI: 10.25259/SNI_836_2021
Clinically useful tumor fluorescence greater than 24 hours after 5-aminolevulinic acid administration
Abstract
Background: 5-aminolevulinic acid (5-ALA) is a valuable surgical adjuvant used for the resection of glioblastoma multiforme (GBM). Since Food and Drug Administration approval in 2017, 5-ALA has been used in over 37,000 cases. The current recommendation for peak efficacy and intraoperative fluorescence is within 4 h after administration. This narrow time window imposes a perioperative time constraint which may complicate or preclude the use of 5-ALA in GBM surgery.
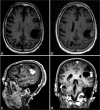
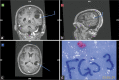
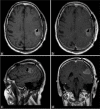
Case description: This case report describes the prolonged activity of 5-ALA in a 66-year-old patient with a newly diagnosed GBM lesion within the left supramarginal gyrus. An awake craniotomy with language and sensorimotor mapping was planned along with 5-ALA fluorescence guidance. Shortly, after receiving the preoperative 5-ALA dose, the patient developed a fever. Surgery was postponed for an infectious disease workup which proved negative. The patient was taken to surgery the following day, 36 h after 5-ALA administration. Despite the delay, intraoperative fluorescence within the tumor remained and was sufficient to guide resection. Postoperative imaging confirmed a gross total resection of the tumor.
Conclusion: The use of 5-ALA as an intraoperative adjuvant may still be effective for patients beyond the recommended 4-h window after initial administration. Reconsideration of current use of 5-ALA is warranted.
Keywords: 5-ALA; 5-Aminolevulinic acid; GBM; Glioblastoma multiforme.
Copyright: © 2022 Surgical Neurology International.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Systematic histopathological analysis of different 5-aminolevulinic acid-induced fluorescence levels in newly diagnosed glioblastomas.J Neurosurg. 2018 Aug;129(2):341-353. doi: 10.3171/2017.4.JNS162991. Epub 2017 Oct 27. J Neurosurg. 2018. PMID: 29076783
-
A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas.J Neurosurg. 2016 May;124(5):1300-9. doi: 10.3171/2015.5.JNS1577. Epub 2015 Nov 6. J Neurosurg. 2016. PMID: 26544781 Clinical Trial.
-
5-aminolevulinic acid guidance during awake craniotomy to maximise extent of safe resection of glioblastoma multiforme.BMJ Case Rep. 2015 Jul 15;2015:bcr2014208575. doi: 10.1136/bcr-2014-208575. BMJ Case Rep. 2015. PMID: 26177997 Free PMC article.
-
Survival Outcomes Among Patients With High-Grade Glioma Treated With 5-Aminolevulinic Acid-Guided Surgery: A Systematic Review and Meta-Analysis.Front Oncol. 2019 Jul 17;9:620. doi: 10.3389/fonc.2019.00620. eCollection 2019. Front Oncol. 2019. PMID: 31380272 Free PMC article.
-
5-ALA fluorescence-assisted surgery in pediatric brain tumors: report of three cases and review of the literature.Br J Neurosurg. 2014 Dec;28(6):750-4. doi: 10.3109/02688697.2014.913779. Epub 2014 May 5. Br J Neurosurg. 2014. PMID: 24799277 Review.
References
-
- Broekx S, Weyns F, De Vleeschouwer S. 5-Aminolevulinic acid for recurrent malignant gliomas: A systematic review. Clin Neurol Neurosurg. 2020;195:105913. - PubMed
-
- Cantrell JN, Waddle MR, Rotman M, Peterson JL, Ruiz-Garcia H, Heckman MG, et al. Progress toward long-term survivors of glioblastoma. Mayo Clin Proc. 2019;94:1278–86. - PubMed
-
- Cozzens JW, Lokaitis BC, Moore BE, Amin DV, Espinosa JA, MacGregor M, et al. A phase 1 dose-escalation study of oral 5-aminolevulinic acid in adult patients undergoing resection of a newly diagnosed or recurrent high-grade glioma. Neurosurgery. 2017;81:46–55. - PubMed
-
- Díez Valle R, Hadjipanayis CG, Stummer W. Established and emerging uses of 5-ALA in the brain: An overview. J Neurooncol. 2019;141:487–94. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
