Persistent Lower Limb Deformities Despite Amelioration of Rickets in X-Linked Hypophosphatemia (XLH) - A Prospective Observational Study
- PMID: 35399930
- PMCID: PMC8987359
- DOI: 10.3389/fendo.2022.866170
Persistent Lower Limb Deformities Despite Amelioration of Rickets in X-Linked Hypophosphatemia (XLH) - A Prospective Observational Study
Abstract
Background: Gait deviations, lower limb pain and joint stiffness represent key symptoms in patients with X-linked hypophosphatemia (XLH, OMIM 307800), a rare disorder of mineral homeostasis. While the pathomechanism for rickets is well understood, the direct role of PHEX (Phosphate-regulating neutral endopeptidase) deficiency in non-rachitic features including complex deformities, skull and dental affections remains unclear. FGF23-inhibiting antibody treatment can normalize serum phosphate levels and to improve rickets in XLH patients. However, linear growth remains impaired and effects on lower limb deformity and gait are insufficiently studied.
Aims: To characterize and evaluate the course of lower limb deformity in a case series of pediatric XLH patients receiving Burosumab therapy.
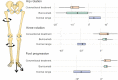
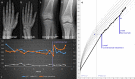
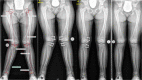
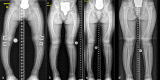
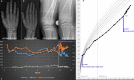
Methods: Comparative assessment of planar radiographs, gait analysis, biochemical and clinical features of pediatric patients before and ≥12 months after initiation of FGF23-inhibiting was performed prospectively. Lower limb maltorsion was quantified by torsional MRI and gait analysis. Standardized deformity analysis of lower limb anteroposterior radiographs was conducted.
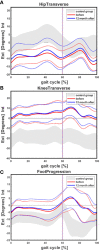
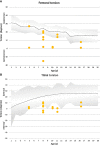
Results: Seven patients (age 9.0 +/-3.6 years) were eligible for this study. All patients received conventional treatment before onset of antibody treatment. Maltorsion of the femur was observed in 8/14 legs using torsional MRI (mean antetorsion 8.79°). Maltorsion of the tibia was observed in 9/14 legs (mean external torsion 2.8°). Gait analysis confirmed MRI findings with femoral external malrotation prior to and one year after onset of Burosumab therapy. Internal foot progression (intoeing gait) remained pathological in all cases (mean 2.2°). Knee rotation was pathologically internal 10/14 legs. Mean mechanical axis deviation (MAD) of 16.1mm prior to Burosumab changed in average by 3.9mm. Three children underwent guided growth procedures within the observation period. Mild postprocedural rebound of frontal axis deviation was observed under Burosumab treatment in one patient.
Conclusions: This is the first study to investigate lower limb deformity parameters quantitatively in children with XLH receiving Burosumab. One year of Burosumab therapy was associated with persistent maltorsion and frontal axis deviation (varus/valgus) despite improved rickets in this small, prospective uncontrolled study.
Keywords: Burosumab; FGF23.; X-linked hypophosphatemia (XLH); deformities; phosphate; skeletal dysplasia; torsion.
Copyright © 2022 Mindler, Stauffer, Kranzl, Penzkofer, Ganger, Radler, Haeusler and Raimann.
Conflict of interest statement
AR and GM received non-related honoraria from Kyowa Kirin for consultancy and scientific presentations. RG and CR received non-related honoraria from Nuvasive Inc. and Smith and Nephew for consultancy. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures


Similar articles
-
Safety and efficacy of burosumab in improving phosphate metabolism, bone health, and quality of life in adolescents with X-linked hypophosphatemic rickets.Eur J Med Genet. 2024 Aug;70:104958. doi: 10.1016/j.ejmg.2024.104958. Epub 2024 Jun 29. Eur J Med Genet. 2024. PMID: 38950880
-
Lower limb maltorsion and acetabular deformity in children and adolescents with X-linked hypophosphatemia.Front Endocrinol (Lausanne). 2024 Sep 19;15:1422356. doi: 10.3389/fendo.2024.1422356. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39371933 Free PMC article.
-
X-linked hypophosphatemia and burosumab: Practical clinical points from the French experience.Joint Bone Spine. 2021 Oct;88(5):105208. doi: 10.1016/j.jbspin.2021.105208. Epub 2021 Jun 6. Joint Bone Spine. 2021. PMID: 34102329
-
Effects of Burosumab Treatment on Two Siblings with X-Linked Hypophosphatemia. Case Report and Literature Review.Genes (Basel). 2022 Aug 4;13(8):1392. doi: 10.3390/genes13081392. Genes (Basel). 2022. PMID: 36011303 Free PMC article. Review.
-
What are the benefits of the anti-FGF23 antibody burosumab on the manifestations of X-linked hypophosphatemia in adults in comparison with conventional therapy? A review.Ther Adv Rare Dis. 2022 Feb 21;3:26330040221074702. doi: 10.1177/26330040221074702. eCollection 2022 Jan-Dec. Ther Adv Rare Dis. 2022. PMID: 37180412 Free PMC article. Review.
Cited by
-
The ankle in XLH: Reduced motion, power and quality of life.Front Endocrinol (Lausanne). 2023 Mar 22;14:1111104. doi: 10.3389/fendo.2023.1111104. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37033213 Free PMC article.
-
Unusual PHEX variants implicate uncommon genetic mechanisms for X-linked hypophosphatemic rickets.JBMR Plus. 2024 Nov 19;9(1):ziae152. doi: 10.1093/jbmrpl/ziae152. eCollection 2025 Jan. JBMR Plus. 2024. PMID: 39677929 Free PMC article.
-
Determinants of Surgical Response to Lateral Tibial Hemiepiphysiodesis in Idiopathic and Non-Idiopathic Genu Varum: Real-World Evidence from a Tertiary Pediatric Cohort.J Clin Med. 2025 Aug 12;14(16):5706. doi: 10.3390/jcm14165706. J Clin Med. 2025. PMID: 40869537 Free PMC article.
-
What's new in pediatric lower limb reconstruction?J Child Orthop. 2024 Jun 12;18(4):349-359. doi: 10.1177/18632521241258351. eCollection 2024 Aug. J Child Orthop. 2024. PMID: 39100980 Free PMC article.
-
Impact of burosumab on lower limb alignment in children with X-linked hypophosphatemia.J Pediatr Soc North Am. 2024 Feb 28;6:100012. doi: 10.1016/j.jposna.2024.100012. eCollection 2024 Feb. J Pediatr Soc North Am. 2024. PMID: 40433251 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

