Remarkable Response to the Triplet Combination of Olaparib with Pembrolizumab and Bevacizumab in the Third-Line Treatment of an Ovarian Clear Cell Carcinoma Patient with an ARID1A Mutation: A Case Report
- PMID: 35401005
- PMCID: PMC8985820
- DOI: 10.2147/OTT.S362267
Remarkable Response to the Triplet Combination of Olaparib with Pembrolizumab and Bevacizumab in the Third-Line Treatment of an Ovarian Clear Cell Carcinoma Patient with an ARID1A Mutation: A Case Report
Abstract
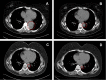
Ovarian clear cell carcinoma (OCCC) is a highly aggressive malignancy with a poor prognosis, and most patients experience recurrence after primary treatment. Currently, there is no standard treatment option for recurrent OCCC. Herein, we report the case of a 32-year-old female patient with OCCC. The patient received primary cytoreductive surgery with adjuvant chemotherapy and remained disease-free for four months. She then experienced retroperitoneal lymph node recurrence and was treated with liposomal doxorubicin chemotherapy followed by secondary debulking surgery. The patient experienced a second relapse in the lower left lung 11 months later. Genomic profiling of tumor samples revealed a deleterious AT-rich interactive domain 1A (ARID1A) mutation and homologous recombination deficiency. Thus, the triplet combination of the poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib; the PD-1 inhibitor, pembrolizumab; and the antiangiogenic agent, bevacizumab was administered. The patient achieved partial response, which was sustained for 12 months. Our study provides the first clinical evidence that the combination of olaparib with pembrolizumab and bevacizumab could be an effective treatment for patients with platinum-resistant, recurrent OCCC.
Keywords: PARP inhibitor; PD-1 inhibitor; antiangiogenic drug; next-generation sequencing; ovarian clear cell carcinoma.
© 2022 Zhao and Jiang.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

