Mitigating Effect of Estrogen in Alzheimer's Disease-Mimicking Cerebral Organoid
- PMID: 35401074
- PMCID: PMC8990972
- DOI: 10.3389/fnins.2022.816174
Mitigating Effect of Estrogen in Alzheimer's Disease-Mimicking Cerebral Organoid
Abstract
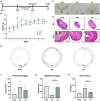
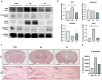
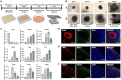
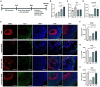
Alzheimer's disease (AD) is the most common condition in patients with dementia and affects a large population worldwide. The incidence of AD is expected to increase in future owing to the rapid expansion of the aged population globally. Researchers have shown that women are twice more likely to be affected by AD than men. This phenomenon has been attributed to the postmenopausal state, during which the level of estrogen declines significantly. Estrogen is known to alleviate neurotoxicity in the brain and protect neurons. While the effects of estrogen have been investigated in AD models, to our knowledge, they have not been investigated in a stem cell-based three-dimensional in vitro system. Here, we designed a new model for AD using induced pluripotent stem cells (iPSCs) in a three-dimensional, in vitro culture system. We used 5xFAD mice to confirm the potential of estrogen in alleviating the effects of AD pathogenesis. Next, we confirmed a similar trend in an AD model developed using iPSC-derived cerebral organoids, in which the key characteristics of AD were recapitulated. The findings emphasized the potential of estrogen as a treatment agent for AD and also showed the suitability of AD-recapitulating cerebral organoids as a reliable platform for disease modeling and drug screening.
Keywords: Alzheimer’s disease; amyloid-beta; cerebral organoid; estrogen; induced pluripotent stem cells.
Copyright © 2022 Kim, Mo, Kim, Kim, Nam, Rim and Ju.
Conflict of interest statement
JK and YN are employed by YiPSCELL, Inc. JHJ is founder of YiPSCELL, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alzamora R., Brown L. R., Harvey B. J. (2007). Direct binding and activation of protein kinase C isoforms by aldosterone and 17β-estradiol. Mole. Endocrinol. 21 2637–2650. - PubMed
LinkOut - more resources
Full Text Sources

