Imaging Synaptic Density: The Next Holy Grail of Neuroscience?
- PMID: 35401097
- PMCID: PMC8990757
- DOI: 10.3389/fnins.2022.796129
Imaging Synaptic Density: The Next Holy Grail of Neuroscience?
Abstract
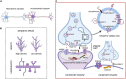
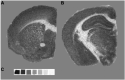
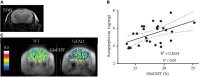
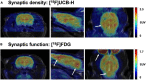
The brain is the central and most complex organ in the nervous system, comprising billions of neurons that constantly communicate through trillions of connections called synapses. Despite being formed mainly during prenatal and early postnatal development, synapses are continually refined and eliminated throughout life via complicated and hitherto incompletely understood mechanisms. Failure to correctly regulate the numbers and distribution of synapses has been associated with many neurological and psychiatric disorders, including autism, epilepsy, Alzheimer's disease, and schizophrenia. Therefore, measurements of brain synaptic density, as well as early detection of synaptic dysfunction, are essential for understanding normal and abnormal brain development. To date, multiple synaptic density markers have been proposed and investigated in experimental models of brain disorders. The majority of the gold standard methodologies (e.g., electron microscopy or immunohistochemistry) visualize synapses or measure changes in pre- and postsynaptic proteins ex vivo. However, the invasive nature of these classic methodologies precludes their use in living organisms. The recent development of positron emission tomography (PET) tracers [such as (18F)UCB-H or (11C)UCB-J] that bind to a putative synaptic density marker, the synaptic vesicle 2A (SV2A) protein, is heralding a likely paradigm shift in detecting synaptic alterations in patients. Despite their limited specificity, novel, non-invasive magnetic resonance (MR)-based methods also show promise in inferring synaptic information by linking to glutamate neurotransmission. Although promising, all these methods entail various advantages and limitations that must be addressed before becoming part of routine clinical practice. In this review, we summarize and discuss current ex vivo and in vivo methods of quantifying synaptic density, including an evaluation of their reliability and experimental utility. We conclude with a critical assessment of challenges that need to be overcome before successfully employing synaptic density biomarkers as diagnostic and/or prognostic tools in the study of neurological and neuropsychiatric disorders.
Keywords: GluCEST; PET; SV2A; electron microscopy; immunohistochemistry; synaptic density.
Copyright © 2022 Serrano, Kim, Petrinovic, Turkheimer and Cash.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

