Cell Type-Specific Transcriptional Control of Gsk3β in the Developing Mammalian Neocortex
- PMID: 35401100
- PMCID: PMC8983961
- DOI: 10.3389/fnins.2022.811689
Cell Type-Specific Transcriptional Control of Gsk3β in the Developing Mammalian Neocortex
Abstract
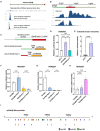
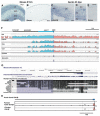
Temporal control of neurogenesis is central for the development and evolution of species-specific brain architectures. The balance between progenitor expansion and neuronal differentiation is tightly coordinated by cell-intrinsic and cell-extrinsic cues. Wnt signaling plays pivotal roles in the proliferation and differentiation of neural progenitors in a temporal manner. However, regulatory mechanisms that adjust intracellular signaling amplitudes according to cell fate progression remain to be elucidated. Here, we report the transcriptional controls of Gsk3β, a critical regulator of Wnt signaling, in the developing mouse neocortex. Gsk3β expression was higher in ventricular neural progenitors, while it gradually declined in differentiated neurons. We identified active cis-regulatory module (CRM) of Gsk3β that responded to cell type-specific transcription factors, such as Sox2, Sox9, and Neurogenin2. Furthermore, we found extensive conservation of the CRM among mammals but not in non-mammalian amniotes. Our data suggest that a mammalian-specific CRM drives the cell type-specific activity of Gsk3β to fine tune Wnt signaling, which contributes to the tight control of neurogenesis during neocortical development.
Keywords: Gsk3β; Wnt signaling; evolution; neocortex; neurogenesis; promoter.
Copyright © 2022 Nomura, Gotoh, Kiyonari and Ono.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Alon L. T., Pietrokovski S., Barkan S., Avrahami L., Kaidanovich-Beilin O., Woodgett J. R., et al. (2011). Selective loss of glycogen synthase kinase-3alpha in birds reveals distinct roles for GSK-3 isozymes in tau phosphorylation. FEBS Lett. 585 1158–1162. 10.1016/j.febslet.2011.03.025 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

