The cAMP Response Element- Binding Protein/Brain-Derived Neurotrophic Factor Pathway in Anterior Cingulate Cortex Regulates Neuropathic Pain and Anxiodepression Like Behaviors in Rats
- PMID: 35401106
- PMCID: PMC8987281
- DOI: 10.3389/fnmol.2022.831151
The cAMP Response Element- Binding Protein/Brain-Derived Neurotrophic Factor Pathway in Anterior Cingulate Cortex Regulates Neuropathic Pain and Anxiodepression Like Behaviors in Rats
Abstract
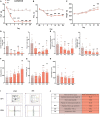
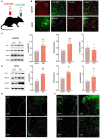
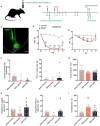
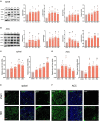
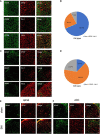
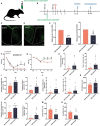
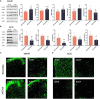
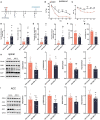
Neuropathic pain is often accompanied by anxiety and depression-like manifestations. Many studies have shown that alterations in synaptic plasticity in the anterior cingulate cortex (ACC) play a critical role, but the specific underlying mechanisms remain unclear. Previously, we showed that cAMP response element-binding protein (CREB) in the dorsal root ganglion (DRG) acts as a transcription factor contributing to neuropathic pain development. At the same time, brain-derived neurotrophic factor (BDNF), as important targets of CREB, is intricate in neuronal growth, differentiation, as well as the establishment of synaptic plasticity. Here, we found that peripheral nerve injury activated the spinal cord and ACC, and silencing the ACC resulted in significant relief of pain sensitivity, anxiety, and depression in SNI rats. In parallel, the CREB/BDNF pathway was activated in the spinal cord and ACC. Central specific knockdown and peripheral non-specific inhibition of CREB reversed pain sensitivity and anxiodepression induced by peripheral nerve injury. Consequently, we identified cingulate CREB/BDNF as an assuring therapeutic method for treating neuropathic pain as well as related anxiodepression.
Keywords: BDNF; CREB; anterior cingulate cortex; anxiety; depression; neuropathic pain.
Copyright © 2022 Wen, Xu, Yu, Zhou, Wang, Yang, Wang, Bai and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Activation of 5-HT6 Receptors in the Ventrolateral Orbital Cortex Produces Anti-Anxiodepressive Effects in a Rat Model of Neuropathic Pain.Mol Neurobiol. 2025 Jan;62(1):1136-1150. doi: 10.1007/s12035-024-04314-1. Epub 2024 Jul 4. Mol Neurobiol. 2025. PMID: 38963532
-
Downregulated spinal IRF8 and BDNF in NAC are involved in neuropathic pain-induced depression relief via pulsed radiofrequency on dorsal root ganglion in rat SNI model.Brain Res Bull. 2019 Mar;146:192-200. doi: 10.1016/j.brainresbull.2019.01.008. Epub 2019 Jan 9. Brain Res Bull. 2019. PMID: 30639279
-
The effect of electroacupuncture on regulating pain and depression-like behaviors induced by chronic neuropathic pain.Ann Palliat Med. 2021 Jan;10(1):104-113. doi: 10.21037/apm-20-1900. Epub 2021 Jan 15. Ann Palliat Med. 2021. PMID: 33474954
-
Cellular, molecular, and epigenetic mechanisms in non-associative conditioning: implications for pain and memory.Neurobiol Learn Mem. 2013 Oct;105:133-50. doi: 10.1016/j.nlm.2013.06.008. Epub 2013 Jun 22. Neurobiol Learn Mem. 2013. PMID: 23796633 Free PMC article. Review.
-
Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex.J Neurochem. 2017 May;141(4):486-498. doi: 10.1111/jnc.14001. Epub 2017 Mar 27. J Neurochem. 2017. PMID: 28251660 Review.
Cited by
-
Proteomic signatures and predictive modeling of cadmium-associated anxiety in middle-aged and elderly populations: an environmental exposure association study.J Transl Med. 2025 May 1;23(1):499. doi: 10.1186/s12967-025-06466-7. J Transl Med. 2025. PMID: 40312359 Free PMC article.
-
Resveratrol Ameliorates Trigeminal Neuralgia-Induced Cognitive Deficits by Regulating Neural Ultrastructural Remodelling and the CREB/BDNF Pathway in Rats.Oxid Med Cell Longev. 2022 Nov 28;2022:4926678. doi: 10.1155/2022/4926678. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36478990 Free PMC article.
-
Evaluating cytidine, uridine, and gabapentin combinations for pain modulation and p-CREB expression in neuropathic model.Future Sci OA. 2025 Dec;11(1):2483137. doi: 10.1080/20565623.2025.2483137. Epub 2025 Mar 31. Future Sci OA. 2025. PMID: 40162624 Free PMC article.
-
Environmental enrichment alleviates neuropathic pain-associated anxiety by enhancing the function of parvalbumin interneurons in the anterior cingulate cortex.Sci Rep. 2025 Mar 24;15(1):10131. doi: 10.1038/s41598-025-95220-6. Sci Rep. 2025. PMID: 40128579 Free PMC article.
-
RNA sequencing profiling of mRNAs, long noncoding RNAs, and circular RNAs in Trigeminal Ganglion following Temporomandibular Joint inflammation.Front Cell Dev Biol. 2022 Aug 16;10:945793. doi: 10.3389/fcell.2022.945793. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36051440 Free PMC article.
References
-
- Boccard S., Fitzgerald J., Pereira E., Moir L., Van Hartevelt T., Kringelbach M., et al. (2014). Targeting the affective component of chronic pain: a case series of deep brain stimulation of the anterior cingulate cortex. Neurosurgery 74 628–635; discussion 635–627. 10.1227/NEU.0000000000000321 - DOI - PubMed
LinkOut - more resources
Full Text Sources

