Identification of Neuronal Cells in Sciatic Nerves of Adult Rats
- PMID: 35401123
- PMCID: PMC8991689
- DOI: 10.3389/fncel.2022.816814
Identification of Neuronal Cells in Sciatic Nerves of Adult Rats
Abstract
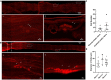
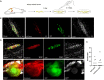
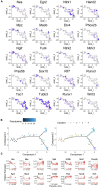
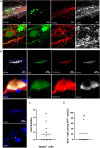
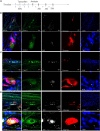
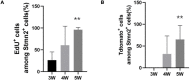
Prior research generally confirms that there are no neuronal cell bodies in the adult sciatic nerve. However, we occasionally find some neuronal cells in adult rat sciatic nerves, either intact or crush-injured. By whole-mount staining and optical imaging of the hyalinized sciatic nerves for Stmn2 (a specific marker for neuronal cells), we found those neuronal cells with irregular distribution in the sciatic nerves in both crushed model and normal rats. We investigated the identity of those cells and established a cultured sciatic nerve model. Immunohistochemistry evidence both in vivo and in vitro illustrated that some of those cells are mature neurons in sciatic nerves. With single-cell sequencing of neuronal cells in adeno-associated virus (AAV)-infected sciatic nerves, we identified that some of those cells are a kind of neuronal stem-like cells. Then we constructed a Nestin-CreERT 2 rat line and traced those cells with fluorescence labeling which was induced by tamoxifen. Interesting, we proved that neuronal stem-like cells could proliferate by combination of EdU incorporation with staining in the sciatic nerves of transgenic rats. Together, the discovery of neuronal cells in adult sciatic nerves will make us aware of the distribution of neurons in the peripheral nervous system. Especially our data suggest that neuronal stem-like cells could proliferate in the sciatic nerves of adult rats.
Keywords: crushed sciatic nerve; neuronal cell bodies; neuronal stem-like cells; sciatic nerve culture; single-cell sequencing; transgenic rat model.
Copyright © 2022 Liu, Zhou, Zhao and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Magnetic resonance imaging evaluation of acute crush injury of rabbit sciatic nerve: correlation with histology.Can Assoc Radiol J. 2008 Jun;59(3):123-30. Can Assoc Radiol J. 2008. PMID: 18697718
-
In vivo evaluation of sciatic nerve crush injury using diffusion tensor imaging: correlation with nerve function and histology.J Comput Assist Tomogr. 2014 Sep-Oct;38(5):790-6. doi: 10.1097/RCT.0000000000000118. J Comput Assist Tomogr. 2014. PMID: 24943253
-
A reassessment of the accuracy of reinnervation by motoneurons following crushing or freezing of the sciatic or lumbar spinal nerves of rats.Brain. 1987 Jun;110 ( Pt 3):695-705. doi: 10.1093/brain/110.3.695. Brain. 1987. PMID: 3580830
-
Quiescent pluripotent stem cells reside within murine peripheral nerves that can be stimulated to proliferate by recombinant human bone morphogenic protein 2 or by nerve trauma.Spine J. 2017 Feb;17(2):252-259. doi: 10.1016/j.spinee.2016.09.016. Epub 2016 Sep 21. Spine J. 2017. PMID: 27664339
-
The growth-associated protein GAP-43 appears in dorsal root ganglion cells and in the dorsal horn of the rat spinal cord following peripheral nerve injury.Neuroscience. 1990;34(2):465-78. doi: 10.1016/0306-4522(90)90155-w. Neuroscience. 1990. PMID: 2139720
Cited by
-
Hybrid construction of tissue-engineered nerve graft using skin derived precursors induced neurons and Schwann cells to enhance peripheral neuroregeneration.Mater Today Bio. 2024 Aug 9;28:101196. doi: 10.1016/j.mtbio.2024.101196. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39221212 Free PMC article.
-
EZH2-dependent myelination following sciatic nerve injury.Neural Regen Res. 2025 Aug 1;20(8):2382-2394. doi: 10.4103/NRR.NRR-D-23-02040. Epub 2024 May 13. Neural Regen Res. 2025. PMID: 39359095 Free PMC article.
References
LinkOut - more resources
Full Text Sources

