Adult Expression of Tbr2 Is Required for the Maintenance but Not Survival of Intrinsically Photosensitive Retinal Ganglion Cells
- PMID: 35401124
- PMCID: PMC8983909
- DOI: 10.3389/fncel.2022.826590
Adult Expression of Tbr2 Is Required for the Maintenance but Not Survival of Intrinsically Photosensitive Retinal Ganglion Cells
Abstract
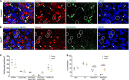
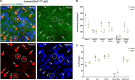
Retinal ganglion cells expressing the photopigment melanopsin are intrinsically photosensitive (ipRGCs). ipRGCs regulate subconscious non-image-forming behaviors such as circadian rhythms, pupil dilation, and light-mediated mood. Previously, we and others showed that the transcription factor Tbr2 (EOMES) is required during retinal development for the formation of ipRGCs. Tbr2 is also expressed in the adult retina leading to the hypothesis that it plays a role in adult ipRGC function. To test this, we removed Tbr2 in adult mice. We found that this results in the loss of melanopsin expression in ipRGCs but does not lead to cell death or morphological changes to their dendritic or axonal termination patterns. Additionally, we found ectopic expression of Tbr2 in conventional RGCs does not induce melanopsin expression but can increase melanopsin expression in existing ipRGCs. An interesting feature of ipRGCs is their superior survival relative to conventional RGCs after an optic nerve injury. We find that loss of Tbr2 decreases the survival rate of ipRGCs after optic nerve damage suggesting that Tbr2 plays a role in ipRGC survival after injury. Lastly, we show that the GABAergic amacrine cell marker Meis2, is expressed in the majority of Tbr2-expressing displaced amacrine cells as well as in a subset of Tbr2-expressing RGCs. These findings demonstrate that Tbr2 is necessary but not sufficient for melanopsin expression, that Tbr2 is involved in ipRGC survival after optic nerve injury, and identify a marker for Tbr2-expressing displaced amacrine cells.
Keywords: EOMES; cell maintenance; melanopsin; optic nerve crush; transcription factor.
Copyright © 2022 Abed, Reilly, Arnold and Feldheim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Agorastos A., Skevas C., Matthaei M., Otte C., Klemm M., Richard G., et al. (2013). Depression, Anxiety, and Disturbed Sleep in Glaucoma. J. Neuropsychiatry Clin. Neurosci. 25 205–213. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

