Long-Term Social Isolation-Induced Autophagy Inhibition and Cell Senescence Aggravate Cognitive Impairment in D(+)Galactose-Treated Male Mice
- PMID: 35401146
- PMCID: PMC8988191
- DOI: 10.3389/fnagi.2022.777700
Long-Term Social Isolation-Induced Autophagy Inhibition and Cell Senescence Aggravate Cognitive Impairment in D(+)Galactose-Treated Male Mice
Abstract
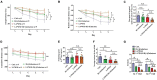
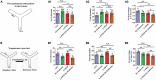
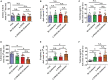
Aging is associated with physiological and pathological changes and presents health complications, such as dementia. Isolation has also been associated with the experience of growing old. Both have been linked individually to the incidence of cognitive decline. In this present study, the effects of these two phenomena have been looked at in animal models where aging was induced with D(+)Galactose in mice who underwent long-term post-weaned social isolation (L-PWSI). Assessing cognitive function using Y-maze, Morris water maze (MWM), and passive avoidance tests (PATs) confirmed that cognition is impaired in either of the treatments but worsened when the D(+)Galactose mice were subjected to L-PWSI. Moreover, a synaptic protein, PSD95, and dendritic spines density were significantly reduced in the L-PWSI and D(+)Galactose-treated mice. Our previous study revealed that autophagy deficit is involved in cognitive impairment in the L-PWSI model. Here, we first report the inhibited cell cycle in L-PWSI, combined with the decreased autophagy, aggravates cognitive impairment in D(+)Galactose-treated mice. Beyond these, the autophagy and cell cycle mechanisms that link isolation and aging have been explored. The close association between isolation and aging in humans is very real and needs much research attention going forward for possible therapeutic interventions.
Keywords: D(+)Galactose; aging; autophagy; cell cycle; cognition; long-term post-weaned social isolation; memory.
Copyright © 2022 Wang, Ntim, Xia, Wang, Lu, Yang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

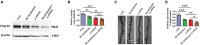

References
-
- Choi Y. J., Anders L. (2014). Signaling through cyclin D-dependent kinases. Oncogene 33 1890–1903. - PubMed
LinkOut - more resources
Full Text Sources

