Palmitic Acid, A Critical Metabolite, Aggravates Cellular Senescence Through Reactive Oxygen Species Generation in Kawasaki Disease
- PMID: 35401162
- PMCID: PMC8983937
- DOI: 10.3389/fphar.2022.809157
Palmitic Acid, A Critical Metabolite, Aggravates Cellular Senescence Through Reactive Oxygen Species Generation in Kawasaki Disease
Abstract
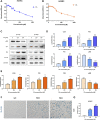
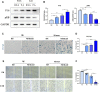
Coronary artery lesions (CALs) are severe complications of Kawasaki disease (KD), resulting in stenosis and thrombogenesis. Metabolomic profiling of patients' plasma could assist in elucidating the pathogenesis of CALs and identifying diagnostic biomarkers, which are imperative for clinical treatment. The metabolic profiles between KD patients with CALs and without CALs (non-coronary artery lesion, or NCAL, group) indicated the most significantly differentially expressed metabolite, palmitic acid (PA), showed the most massive fold change at 9.879. Furthermore, PA was proven to aggravate endothelial cellular senescence by increasing the generation of reactive oxygen species (ROS) in KD, and those two phenotypes were confirmed to be enriched among the differentially expressed genes between KD and normal samples from GEO datasets. Collectively, our findings indicate that cellular senescence may be one of the mechanisms of vascular endothelial damage in KD. PA may be a biomarker and potential therapeutic target for predicting the occurrence of CALs in KD patients. All things considered, our findings confirm that plasma metabolomics was able to identify promising biomarkers and potential pathogenesis mechanisms in KD. To conclude, Palmitic acid could be a novel target in future studies of CALs in patients with KD.
Keywords: cellular senescence; coronary disease; kawasaki disease; metabolomics; oxidative stress.
Copyright © 2022 Zhu, Dong, Wang, Xia, Fu, Wang, Wu and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Facial nerve palsy may indicate coronary artery lesions in Kawasaki disease.Clin Rheumatol. 2021 Oct;40(10):4191-4197. doi: 10.1007/s10067-021-05791-8. Epub 2021 May 31. Clin Rheumatol. 2021. PMID: 34059986
-
The role of red blood cell distribution width in predicting coronary artery lesions in pediatric patients with kawasaki disease.Front Cardiovasc Med. 2023 Mar 3;10:1014890. doi: 10.3389/fcvm.2023.1014890. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36937943 Free PMC article.
-
Circulating endothelial glycocalyx components as a predictive marker of coronary artery lesions in Kawasaki disease.Int J Cardiol. 2019 Oct 1;292:236-240. doi: 10.1016/j.ijcard.2019.05.045. Epub 2019 May 22. Int J Cardiol. 2019. PMID: 31200965
-
The Benefits and Respective Side-Effects of PE Therapy for Intractable Kawasaki Disease.J Clin Med. 2021 Mar 4;10(5):1062. doi: 10.3390/jcm10051062. J Clin Med. 2021. PMID: 33806522 Free PMC article. Review.
-
State-of-the-art acute phase management of Kawasaki disease after 2017 scientific statement from the American Heart Association.Pediatr Neonatol. 2018 Dec;59(6):543-552. doi: 10.1016/j.pedneo.2018.03.005. Epub 2018 Mar 30. Pediatr Neonatol. 2018. PMID: 29706362 Review.
Cited by
-
Targeting Insulin Resistance, Reactive Oxygen Species, Inflammation, Programmed Cell Death, ER Stress, and Mitochondrial Dysfunction for the Therapeutic Prevention of Free Fatty Acid-Induced Vascular Endothelial Lipotoxicity.Antioxidants (Basel). 2024 Dec 5;13(12):1486. doi: 10.3390/antiox13121486. Antioxidants (Basel). 2024. PMID: 39765815 Free PMC article. Review.
-
FCGR2/3 polymorphisms are associated with susceptibility to Kawasaki disease but do not predict intravenous immunoglobulin resistance and coronary artery aneurysms.Front Immunol. 2024 Sep 18;15:1323171. doi: 10.3389/fimmu.2024.1323171. eCollection 2024. Front Immunol. 2024. PMID: 39359734 Free PMC article.
-
Metabolomics-driven approaches for identifying therapeutic targets in drug discovery.MedComm (2020). 2024 Nov 11;5(11):e792. doi: 10.1002/mco2.792. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39534557 Free PMC article. Review.
-
Biologically informed machine learning modeling of immune cells to reveal physiological and pathological aging process.Immun Ageing. 2024 Oct 24;21(1):74. doi: 10.1186/s12979-024-00479-4. Immun Ageing. 2024. PMID: 39449067 Free PMC article.
-
Identification of novel metabolism-related biomarkers of Kawasaki disease by integrating single-cell RNA sequencing analysis and machine learning algorithms.Front Immunol. 2025 Apr 10;16:1541939. doi: 10.3389/fimmu.2025.1541939. eCollection 2025. Front Immunol. 2025. PMID: 40276515 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

