Fraxin Promotes the Activation of Nrf2/ARE Pathway via Increasing the Expression of Connexin43 to Ameliorate Diabetic Renal Fibrosis
- PMID: 35401165
- PMCID: PMC8987976
- DOI: 10.3389/fphar.2022.853383
Fraxin Promotes the Activation of Nrf2/ARE Pathway via Increasing the Expression of Connexin43 to Ameliorate Diabetic Renal Fibrosis
Abstract
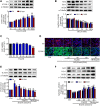
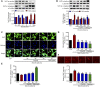
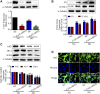
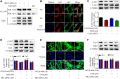
Diabetic nephropathy (DN) is quickly becoming the largest cause of end-stage renal disease (ESRD) in diabetic patients, as well as a major source of morbidity and mortality. Our previous studies indicated that the activation of Nrf2/ARE pathway via Connexin43 (Cx43) considerably contribute to the prevention of oxidative stress in the procession of DN. Fraxin (Fr), the main active glycoside of Fraxinus rhynchophylla Hance, has been demonstrated to possess many potential pharmacological activities. Whereas, whether Fr could alleviate renal fibrosis through regulating Cx43 and consequently facilitating the activation of Nrf2/ARE pathway needs further investigation. The in vitro results showed that: 1) Fr increased the expression of antioxidant enzymes including SOD1 and HO-1 to inhibit high glucose (HG)-induced fibronectin (FN) and inflammatory cell adhesion molecule (ICAM-1) overexpression; 2) Fr exerted antioxidant effect through activating the Nrf2/ARE pathway; 3) Fr significantly up-regulated the expression of Cx43 in HG-induced glomerular mesangial cells (GMCs), while the knock down of Cx43 largely impaired the activation of Nrf2/ARE pathway induced by Fr; 4) Fr promoted the activation of Nrf2/ARE pathway via regulating the interaction between Cx43 and AKT. Moreover, in accordance with the results in vitro, elevated levels of Cx43, phosphorylated-AKT, Nrf2 and downstream antioxidant enzymes related to Nrf2 were observed in the kidneys of Fr-treated group compared with model group. Importantly, Fr significantly improved renal dysfunction pathological changes of renal fibrosis in diabetic db/db mice. Collectively, Fr could increase the Cx43-AKT-Nrf2/ARE pathway activation to postpone the diabetic renal fibrosis and the up-regulation of Cx43 is probably a novel mechanism in this process.
Keywords: AKT; Connecxin43; Nrf2/ARE pathway; diabetic nephropathy; fraxin; oxidative stress.
Copyright © 2022 Chen, Zeng, Li, Xiao, Li, Lin, Huang, Shen and Huang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer CL declared a shared parent affiliation with a number of the authors HH, ZL, SL, HX, CL, JZ, RI at the time of the review.
Figures



Similar articles
-
Polydatin promotes Nrf2-ARE anti-oxidative pathway through activating CKIP-1 to resist HG-induced up-regulation of FN and ICAM-1 in GMCs and diabetic mice kidneys.Free Radic Biol Med. 2017 May;106:393-405. doi: 10.1016/j.freeradbiomed.2017.03.003. Epub 2017 Mar 10. Free Radic Biol Med. 2017. PMID: 28286065
-
Astaxanthin ameliorates experimental diabetes-induced renal oxidative stress and fibronectin by upregulating connexin43 in glomerular mesangial cells and diabetic mice.Eur J Pharmacol. 2018 Dec 5;840:33-43. doi: 10.1016/j.ejphar.2018.09.028. Epub 2018 Sep 27. Eur J Pharmacol. 2018. PMID: 30268666
-
Connexin43 regulates high glucose-induced expression of fibronectin, ICAM-1 and TGF-β1 via Nrf2/ARE pathway in glomerular mesangial cells.Free Radic Biol Med. 2017 Jan;102:77-86. doi: 10.1016/j.freeradbiomed.2016.11.015. Epub 2016 Nov 11. Free Radic Biol Med. 2017. PMID: 27840317
-
Paeonol Ameliorates Diabetic Renal Fibrosis Through Promoting the Activation of the Nrf2/ARE Pathway via Up-Regulating Sirt1.Front Pharmacol. 2018 May 18;9:512. doi: 10.3389/fphar.2018.00512. eCollection 2018. Front Pharmacol. 2018. PMID: 29867511 Free PMC article.
-
CKIP-1 affects the polyubiquitination of Nrf2 and Keap1 via mediating Smurf1 to resist HG-induced renal fibrosis in GMCs and diabetic mice kidneys.Free Radic Biol Med. 2018 Feb 1;115:338-350. doi: 10.1016/j.freeradbiomed.2017.12.013. Epub 2017 Dec 14. Free Radic Biol Med. 2018. PMID: 29248720
Cited by
-
Molecular Mechanistic Pathways Targeted by Natural Compounds in the Prevention and Treatment of Diabetic Kidney Disease.Molecules. 2022 Sep 21;27(19):6221. doi: 10.3390/molecules27196221. Molecules. 2022. PMID: 36234757 Free PMC article. Review.
-
NRF2 Dysregulation and Therapeutic Insights Across Chronic Kidney Diseases.Int J Mol Sci. 2025 Aug 2;26(15):7471. doi: 10.3390/ijms26157471. Int J Mol Sci. 2025. PMID: 40806598 Free PMC article. Review.
References
-
- Ball K. K., Harik L., Gandhi G. K., Cruz N. F., Dienel G. A. (2011). Reduced gap Junctional Communication Among Astrocytes in Experimental Diabetes: Contributions of Altered Connexin Protein Levels and Oxidative-Nitrosative Modifications. J. Neurosci. Res. 89, 2052–2067. 10.1002/jnr.22663 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

