A Comprehensive Review of BET Protein Biochemistry, Physiology, and Pathological Roles
- PMID: 35401196
- PMCID: PMC8990909
- DOI: 10.3389/fphar.2022.818891
A Comprehensive Review of BET Protein Biochemistry, Physiology, and Pathological Roles
Abstract
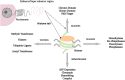
Epigenetic modifications, specifically acetylation of histone plays a decisive role in gene regulation and transcription of normal cellular mechanisms and pathological conditions. The bromodomain and extraterminal (BET) proteins (BRD2, BRD3, BRD4, and BRDT), being epigenetic readers, ligate to acetylated regions of histone and synchronize gene transcription. BET proteins are crucial for normal cellular processing as they control cell cycle progression, neurogenesis, differentiation, and maturation of erythroids and spermatogenesis, etc. Research-based evidence indicated that BET proteins (mainly BRD4) are associated with numeral pathological ailments, including cancer, inflammation, infections, renal diseases, and cardiac diseases. To counter the BET protein-related pathological conditions, there are some BET inhibitors developed and also under development. BET proteins are a topic of most research nowadays. This review, provides an ephemeral but comprehensive knowledge about BET proteins' basic structure, biochemistry, physiological roles, and pathological conditions in which the role of BETs have been proven. This review also highlights the current and future approaches to pledge BET protein-related pathologies.
Keywords: BET inhibitors; BET proteins; BRD4; Histone acetylation; epigenetics.
Copyright © 2022 Ali, Li, Bilal, Qin, Yuan and Zhao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

