Combination Therapy of Alpha-Lipoic Acid, Gliclazide and Ramipril Protects Against Development of Diabetic Cardiomyopathy via Inhibition of TGF-β/Smad Pathway
- PMID: 35401218
- PMCID: PMC8988231
- DOI: 10.3389/fphar.2022.850542
Combination Therapy of Alpha-Lipoic Acid, Gliclazide and Ramipril Protects Against Development of Diabetic Cardiomyopathy via Inhibition of TGF-β/Smad Pathway
Abstract
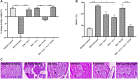
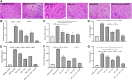
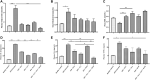
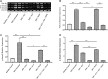
Background: Diabetic cardiomyopathy (DCM) is a major long-term complication of diabetes mellitus, accounting for over 20% of annual mortality rate of diabetic patients globally. Although several existing anti-diabetic drugs have improved glycemic status in diabetic patients, prevalence of DCM is still high. This study investigates cardiac effect of alpha-lipoic acid (ALA) supplementation of anti-diabetic therapy in experimental DCM. Methods: Following 12 h of overnight fasting, 44 male Sprague Dawley rats were randomly assigned to two groups of healthy control (n = 7) and diabetic (n = 37) groups, and fasting blood glucose was measured. Type 2 diabetes mellitus (T2DM) was induced in diabetic group by intraperitoneal (i.p.) administration of nicotinamide (110 mg/kg) and streptozotocin (55 mg/kg). After confirmation of T2DM on day 3, diabetic rats received monotherapies with ALA (60 mg/kg; n = 7), gliclazide (15 mg/kg; n = 7), ramipril (10 mg/kg; n = 7) or combination of the three drugs (n = 7) for 6 weeks while untreated diabetic rats received distilled water and were used as diabetic control (n = 9). Rats were then sacrificed, and blood, pancreas and heart tissues were harvested for analyses using standard methods. Results: T2DM induction caused pancreatic islet destruction, hyperglycemia, weight loss, high relative heart weight, and development of DCM, which was characterized by myocardial degeneration and vacuolation, cardiac fibrosis, elevated cardiac damage markers (plasma and cardiac creatine kinase-myocardial band, brain natriuretic peptide and cardiac troponin I). Triple combination therapy of ALA, gliclazide and ramipril preserved islet structure, maintained body weight and blood glucose level, and prevented DCM development compared to diabetic control (p < 0.001). In addition, the combination therapy markedly reduced plasma levels of inflammatory markers (IL-1β, IL-6 and TNF-α), plasma and cardiac tissue malondialdehyde, triglycerides and total cholesterol while significantly increasing cardiac glutathione and superoxide dismutase activity and high-density lipoprotein-cholesterol compared to diabetic control (p < 0.001). Mechanistically, induction of T2DM upregulated cardiac expression of TGF-β1, phosphorylated Smad2 and Smad3 proteins, which were downregulated following triple combination therapy (p < 0.001). Conclusion: Triple combination therapy of ALA, gliclazide and ramipril prevented DCM development by inhibiting TGF-β1/Smad pathway. Our findings can be extrapolated to the human heart, which would provide effective additional pharmacological therapy against DCM in T2DM patients.
Keywords: alpha-lipoic acid (ALA); anti-diabetic therapy; diabetic cardiomyopathy (DCM); triple combination therapy; type 2 diabetes mellitus (T2DM).
Copyright © 2022 Dugbartey, Wonje, Alornyo, Robertson, Adams, Boima and Mensah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Alpha-lipoic acid treatment improves adverse cardiac remodelling in the diabetic heart - The role of cardiac hydrogen sulfide-synthesizing enzymes.Biochem Pharmacol. 2022 Sep;203:115179. doi: 10.1016/j.bcp.2022.115179. Epub 2022 Jul 16. Biochem Pharmacol. 2022. PMID: 35853498
-
Hepatoprotective potential of alpha-lipoic acid against gliclazide-induced liver injury in high-glucose-exposed human liver cells and experimental type 2 diabetic rats.Biochem Pharmacol. 2024 Sep;227:116447. doi: 10.1016/j.bcp.2024.116447. Epub 2024 Jul 20. Biochem Pharmacol. 2024. PMID: 39038553
-
Supplementation of conventional anti-diabetic therapy with alpha-lipoic acid prevents early development and progression of diabetic nephropathy.Biomed Pharmacother. 2022 May;149:112818. doi: 10.1016/j.biopha.2022.112818. Epub 2022 Mar 11. Biomed Pharmacother. 2022. PMID: 35286963
-
Role of Circulating Biomarkers in Diabetic Cardiomyopathy.Biomedicines. 2024 Sep 23;12(9):2153. doi: 10.3390/biomedicines12092153. Biomedicines. 2024. PMID: 39335666 Free PMC article. Review.
-
The signaling pathways of selected traditional Chinese medicine prescriptions and their metabolites in the treatment of diabetic cardiomyopathy: a review.Front Pharmacol. 2024 Jul 3;15:1416403. doi: 10.3389/fphar.2024.1416403. eCollection 2024. Front Pharmacol. 2024. PMID: 39021834 Free PMC article. Review.
Cited by
-
Targeting hepatic sulfane sulfur/hydrogen sulfide signaling pathway with α-lipoic acid to prevent diabetes-induced liver injury via upregulating hepatic CSE/3-MST expression.Diabetol Metab Syndr. 2022 Oct 13;14(1):148. doi: 10.1186/s13098-022-00921-x. Diabetol Metab Syndr. 2022. PMID: 36229864 Free PMC article.
-
Alpha-lipoic acid: A promising pharmacotherapy seen through the lens of kidney diseases.Curr Res Pharmacol Drug Discov. 2024 Oct 26;7:100206. doi: 10.1016/j.crphar.2024.100206. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 39524210 Free PMC article. Review.
-
The immunology of diabetic cardiomyopathy.Front Endocrinol (Lausanne). 2025 Apr 7;16:1542208. doi: 10.3389/fendo.2025.1542208. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40260277 Free PMC article. Review.
-
Molecular Insights into Oxidative-Stress-Mediated Cardiomyopathy and Potential Therapeutic Strategies.Biomolecules. 2025 May 6;15(5):670. doi: 10.3390/biom15050670. Biomolecules. 2025. PMID: 40427563 Free PMC article. Review.
-
Inflammation in diabetes complications: molecular mechanisms and therapeutic interventions.MedComm (2020). 2024 Apr 12;5(4):e516. doi: 10.1002/mco2.516. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38617433 Free PMC article. Review.
References
-
- Agrawal N. K., Gupta U. (2013). Evaluation of Ramipril on Blood Sugar Level and Interaction with the Oral Anti-diabetic Drugs in Alloxan-Induced Diabetic Rats. Int. J. Pharm. Sci. Res. 4 (8), 2933–2938. 10.13040/IJPSR.0975-8232.4(8).2933-38 - DOI
-
- Ajiboye B. O., Oyinloye B. E., Onikanni S. A., Osukoya O. A., Lawal O. E., Bamisaye F. A. (2021). Sterculia Tragacantha Lindl Aqueous Leaf Extract Ameliorate Cardiomyopathy in Streptozotocin-Induced Diabetic Rats via Urotensin II and FABP3 Expressions. J. Oleo Sci. 70 (12), 1805–1814. 10.5650/jos.ess21251 - DOI - PubMed
-
- Akbari M., Ostadmohammadi V., Lankarani K. B., Tabrizi R., Kolahdooz F., Khatibi S. R., et al. (2018). The Effects of Alpha-Lipoic Acid Supplementation on Glucose Control and Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Metabolism 87, 56–69. 10.1016/j.metabol.2018.07.002 - DOI - PubMed
-
- Aslfalah H., Jamilian M., Rafiei F., Khosrowbeygi A. (2019). Reduction in Maternal Serum Values of Glucose and Gamma-Glutamyltransferase after Supplementation with Alpha-Lipoic Acid in Women with Gestational Diabetes Mellitus. J. Obstet. Gynaecol. Res. 45 (2), 313–317. 10.1111/jog.13842 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

