Drug Prescriptions in the Outpatient Management of COVID-19: Evidence-Based Recommendations Versus Real Practice
- PMID: 35401220
- PMCID: PMC8988061
- DOI: 10.3389/fphar.2022.825479
Drug Prescriptions in the Outpatient Management of COVID-19: Evidence-Based Recommendations Versus Real Practice
Abstract
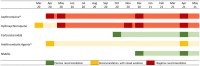
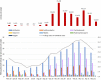
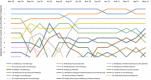
Background: Evidence-based recommendations for outpatient management of COVID-19 were published by the Italian Medicines Agency (AIFA) to limit the use of off-label treatments. The aim of this study is to measure the use of outpatient drug treatments in a COVID-19-positive population, taking into account the Italian regulatory agency's advices. Methods: A descriptive observational study was conducted. All patients testing positive for COVID-19 residing in Lazio region, Italy, with diagnosis date between March 2020 and May 2021 were selected, and outpatient medicine prescription patterns were identified. Results: Independent of AIFA recommendations, the use of drug therapy in the management of outpatient COVID-19 cases was frequent (about one-third of the cases). The most used drug therapy was antibiotics, specifically azithromycin, despite the negative recommendation of AIFA, while the use of corticosteroids increased after the positive recommendation of regulatory agency for the use in subjects with severe COVID-19 disease. The use of hydroxychloroquine was limited to the early pandemic period where evidence on its potential benefit was controversial. Antithrombotics were widely used in outpatient settings, even if their use was recommended for hospitalized patients. Conclusion: In this study, we show a frequent use of drug therapy in the management of outpatient cases of COVID-19, mainly attributable to antibiotics use. Our research highlights the discrepancy between recommendations for care and clinical practice and the need for strategies to bridge gaps in evidence-informed decision-making.
Keywords: COVID-19; drug monitoring; drug prescription; evidence-based medicine; outpatient care.
Copyright © 2022 Belleudi, Finocchietti, Fortinguerra, Di Filippo, Trotta, Davoli and Addis.
Conflict of interest statement
FF, AD, and FT were employed by the Italian Medicines Agency (AIFA), the national authority responsible for the regulatory, pricing and reimbursement (PR), and HTA activities related to pharmaceuticals including governance of pharmaceutical expenditure. The remaining authors declare that the research was conducted in absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Evaluation of outpatient prescription patterns of COVID-19 drugs in Iran: comparison of real practice with local therapeutic recommendations.J Pharm Policy Pract. 2023 Nov 27;16(1):157. doi: 10.1186/s40545-023-00672-8. J Pharm Policy Pract. 2023. PMID: 38012696 Free PMC article.
-
Responsible, Safe, and Effective Prescription of Opioids for Chronic Non-Cancer Pain: American Society of Interventional Pain Physicians (ASIPP) Guidelines.Pain Physician. 2017 Feb;20(2S):S3-S92. Pain Physician. 2017. PMID: 28226332
-
COVID-19 Patient Management in Outpatient Setting: A Population-Based Study from Southern Italy.J Clin Med. 2021 Dec 23;11(1):51. doi: 10.3390/jcm11010051. J Clin Med. 2021. PMID: 35011810 Free PMC article.
-
Impact of the 2014 American Academy of Pediatrics recommendation and of the resulting limited financial coverage by the Italian Medicines Agency for palivizumab prophylaxis on the RSV-associated hospitalizations in preterm infants during the 2016-2017 epidemic season: a systematic review of seven Italian reports.Ital J Pediatr. 2019 Nov 9;45(1):139. doi: 10.1186/s13052-019-0736-5. Ital J Pediatr. 2019. PMID: 31706338 Free PMC article.
-
Electroencephalography during SARS-CoV-2 outbreak: practical recommendations from the task force of the Italian Society of Neurophysiology (SINC), the Italian League Against Epilepsy (LICE), and the Italian Association of Neurophysiology Technologists (AITN).Neurol Sci. 2020 Sep;41(9):2345-2351. doi: 10.1007/s10072-020-04585-1. Epub 2020 Jul 21. Neurol Sci. 2020. PMID: 32696088 Free PMC article. Review.
Cited by
-
Evaluating the Impact of Interventions and Improvements in Outpatient Intravenous Infusion Therapy at a Hospital in China: A Comprehensive Analysis of Prescription Patterns and Safety Measures.Risk Manag Healthc Policy. 2024 Mar 8;17:525-533. doi: 10.2147/RMHP.S451516. eCollection 2024. Risk Manag Healthc Policy. 2024. PMID: 38476200 Free PMC article.
-
Evaluating the utilisation patterns of pharmacological therapy in COVID-19 patients: an ecological study in Italy.BMJ Public Health. 2025 Feb 26;3(1):e001767. doi: 10.1136/bmjph-2024-001767. eCollection 2025. BMJ Public Health. 2025. PMID: 40017974 Free PMC article.
-
Association between self-reported evidence-based medicine competencies and prescribing of drugs without scientific evidence against mild COVID-19 among recently graduated physicians in Peru.Heliyon. 2023 Apr;9(4):e15366. doi: 10.1016/j.heliyon.2023.e15366. Epub 2023 Apr 8. Heliyon. 2023. PMID: 37064449 Free PMC article.
-
Evaluation of outpatient prescription patterns of COVID-19 drugs in Iran: comparison of real practice with local therapeutic recommendations.J Pharm Policy Pract. 2023 Nov 27;16(1):157. doi: 10.1186/s40545-023-00672-8. J Pharm Policy Pract. 2023. PMID: 38012696 Free PMC article.
-
Reduction in broad-spectrum antimicrobial prescriptions by primary care pediatricians following a multifaceted antimicrobial stewardship program.Front Pediatr. 2023 Jan 6;10:1070325. doi: 10.3389/fped.2022.1070325. eCollection 2022. Front Pediatr. 2023. PMID: 36683814 Free PMC article.
References
-
- Ammassari A., Di Filippo A., Trotta M. P., Traversa G., Pierantozzi A., Trotta F., et al. (2021). Comparison of Demand for Drugs Used for COVID-19 Treatment and Other Drugs during the Early Phase of the COVID-19 Pandemic in Italy. JAMA Netw. Open 4 (2), e2037060. 10.1001/jamanetworkopen.2020.37060 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

