Drug Prescriptions in the Outpatient Management of COVID-19: Evidence-Based Recommendations Versus Real Practice
- PMID: 35401220
- PMCID: PMC8988061
- DOI: 10.3389/fphar.2022.825479
Drug Prescriptions in the Outpatient Management of COVID-19: Evidence-Based Recommendations Versus Real Practice
Abstract
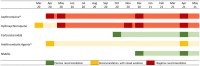
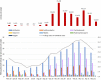
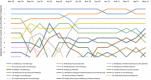
Background: Evidence-based recommendations for outpatient management of COVID-19 were published by the Italian Medicines Agency (AIFA) to limit the use of off-label treatments. The aim of this study is to measure the use of outpatient drug treatments in a COVID-19-positive population, taking into account the Italian regulatory agency's advices. Methods: A descriptive observational study was conducted. All patients testing positive for COVID-19 residing in Lazio region, Italy, with diagnosis date between March 2020 and May 2021 were selected, and outpatient medicine prescription patterns were identified. Results: Independent of AIFA recommendations, the use of drug therapy in the management of outpatient COVID-19 cases was frequent (about one-third of the cases). The most used drug therapy was antibiotics, specifically azithromycin, despite the negative recommendation of AIFA, while the use of corticosteroids increased after the positive recommendation of regulatory agency for the use in subjects with severe COVID-19 disease. The use of hydroxychloroquine was limited to the early pandemic period where evidence on its potential benefit was controversial. Antithrombotics were widely used in outpatient settings, even if their use was recommended for hospitalized patients. Conclusion: In this study, we show a frequent use of drug therapy in the management of outpatient cases of COVID-19, mainly attributable to antibiotics use. Our research highlights the discrepancy between recommendations for care and clinical practice and the need for strategies to bridge gaps in evidence-informed decision-making.
Keywords: COVID-19; drug monitoring; drug prescription; evidence-based medicine; outpatient care.
Copyright © 2022 Belleudi, Finocchietti, Fortinguerra, Di Filippo, Trotta, Davoli and Addis.
Conflict of interest statement
FF, AD, and FT were employed by the Italian Medicines Agency (AIFA), the national authority responsible for the regulatory, pricing and reimbursement (PR), and HTA activities related to pharmaceuticals including governance of pharmaceutical expenditure. The remaining authors declare that the research was conducted in absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ammassari A., Di Filippo A., Trotta M. P., Traversa G., Pierantozzi A., Trotta F., et al. (2021). Comparison of Demand for Drugs Used for COVID-19 Treatment and Other Drugs during the Early Phase of the COVID-19 Pandemic in Italy. JAMA Netw. Open 4 (2), e2037060. 10.1001/jamanetworkopen.2020.37060 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

