Salvia miltiorrhiza Bunge (Danshen) and Bioactive Compound Tanshinone IIA Alleviates Cisplatin-Induced Acute Kidney Injury Through Regulating PXR/NF-κB Signaling
- PMID: 35401224
- PMCID: PMC8987575
- DOI: 10.3389/fphar.2022.860383
Salvia miltiorrhiza Bunge (Danshen) and Bioactive Compound Tanshinone IIA Alleviates Cisplatin-Induced Acute Kidney Injury Through Regulating PXR/NF-κB Signaling
Abstract
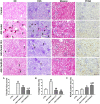
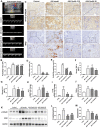
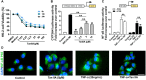
Objective: The present study aims to provide evidence on the potential protective role of Salvia miltiorrhiza Bunge (Danshen) and its bioactive compound Tanshinone IIA (TanIIA) in AKI and to reveal the specific regulatory function of PXR/NF-κB signaling in AKI-induced renal inflammation. Methods: A network pharmacological analysis was used to study target genes and regulatory networks in the treatment of Salvia miltiorrhiza on AKI. Further experiments with in vivo AKI mouse model and in vitro studies were applied to investigate the renal protective effect of TanIIA in AKI. The mechanisms of TanIIA regulating PXR/NF-κB signaling in renal inflammation were also studied. Results: Network pharmacology had suggested the nuclear receptor family as new therapeutic targets of Salvia miltiorrhiza in AKI treatment. The in vivo studies had demonstrated that TanIIA improved renal function and inflammation by reducing necrosis and promoting the proliferation of tubular epithelial cells. Improved renal arterial perfusion in AKI mice with TanIIA treatment was also recorded by ultrasonography. In vitro studies had shown that TanIIA ameliorated renal inflammation by activating the PXR while inhibiting PXR-mediated NF-κB signaling. The results had suggested a role of PXR activation against AKI-induced renal inflammation. Conclusion: Salvia miltiorrhiza Bunge (Danshen) may protect the kidneys against AKI by regulating nuclear receptors. TanIIA improved cell necrosis proliferation and reduced renal inflammation by upregulating the expression of the PXR and inhibiting NF-κB signaling in a PXR-dependent manner. The PXR may be a potential therapeutic target for AKI treatment.
Keywords: NF-κB; Salvia miltiorrhiza; acute kidney injury; pregnane X receptor; renal inflammation; tanshinone IIA.
Copyright © 2022 Dou, Zhang, Cen, Chen, Wu, Lu, Zhou, Liu and Gu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Exploring the Therapeutic Role of Pregnane X Receptor Activation in Acute Kidney Injury: Mechanisms and Clinical Implications.Curr Mol Med. 2025 Jun 12. doi: 10.2174/0115665240381768250607064429. Online ahead of print. Curr Mol Med. 2025. PMID: 40511830
-
PXR-mediated transcriptional activation of CYP3A4 by cryptotanshinone and tanshinone IIA.Chem Biol Interact. 2009 Jan 15;177(1):58-64. doi: 10.1016/j.cbi.2008.08.013. Epub 2008 Sep 2. Chem Biol Interact. 2009. PMID: 18805405
-
Targeting Oxidative Stress and Endothelial Dysfunction Using Tanshinone IIA for the Treatment of Tissue Inflammation and Fibrosis.Oxid Med Cell Longev. 2022 Apr 7;2022:2811789. doi: 10.1155/2022/2811789. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35432718 Free PMC article. Review.
-
Tanshinone IIA Protects Endothelial Cells from H₂O₂-Induced Injuries via PXR Activation.Biomol Ther (Seoul). 2017 Nov 1;25(6):599-608. doi: 10.4062/biomolther.2016.179. Biomol Ther (Seoul). 2017. PMID: 28173640 Free PMC article.
-
Expanding the therapeutic potential of Salvia miltiorrhiza: a review of its pharmacological applications in musculoskeletal diseases.Front Pharmacol. 2023 Dec 5;14:1276038. doi: 10.3389/fphar.2023.1276038. eCollection 2023. Front Pharmacol. 2023. PMID: 38116081 Free PMC article. Review.
Cited by
-
In Silico Analysis of Pyeongwi-San Involved in Inflammatory Bowel Disease Treatment Using Network Pharmacology, Molecular Docking, and Molecular Dynamics.Biomolecules. 2023 Aug 28;13(9):1322. doi: 10.3390/biom13091322. Biomolecules. 2023. PMID: 37759722 Free PMC article.
-
Renoprotective Effects of Tanshinone IIA: A Literature Review.Molecules. 2023 Feb 20;28(4):1990. doi: 10.3390/molecules28041990. Molecules. 2023. PMID: 36838978 Free PMC article. Review.
-
[Tanshinone IIA alleviates lipopolysaccharide-induced renal tubular epithelial cell apoptosis by inhibiting RIP3/FUNDC1 signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2022 Dec 20;42(12):1852-1857. doi: 10.12122/j.issn.1673-4254.2022.12.14. Nan Fang Yi Ke Da Xue Xue Bao. 2022. PMID: 36651254 Free PMC article. Chinese.
-
Research Progress on Regulation of Immune Response by Tanshinones and Salvianolic Acids of Danshen (Salvia miltiorrhiza Bunge).Molecules. 2024 Mar 7;29(6):1201. doi: 10.3390/molecules29061201. Molecules. 2024. PMID: 38542838 Free PMC article. Review.
-
Nephroprotective effects of substances of medicine food homology and traditional Chinese medicine phytochemicals against acute kidney injury.Front Pharmacol. 2025 Feb 19;16:1539886. doi: 10.3389/fphar.2025.1539886. eCollection 2025. Front Pharmacol. 2025. PMID: 40046749 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

