Alpha-Asaronol Alleviates Dysmyelination by Enhancing Glutamate Transport Through the Activation of PPARγ-GLT-1 Signaling in Hypoxia-Ischemia Neonatal Rats
- PMID: 35401225
- PMCID: PMC8984140
- DOI: 10.3389/fphar.2022.766744
Alpha-Asaronol Alleviates Dysmyelination by Enhancing Glutamate Transport Through the Activation of PPARγ-GLT-1 Signaling in Hypoxia-Ischemia Neonatal Rats
Abstract
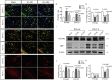
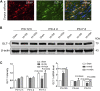
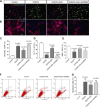
Preterm white matter injury (PWMI) is the most common form of brain damage in premature infants caused by hypoxia-ischemia (HI), inflammation, or excitotoxicity. It is characterized by oligodendrocyte precursor cell (OPC) differentiation disorder and dysmyelination. Our previous study confirmed that alpha-asarone (α-asaronol), a major compound isolated from the Chinese medicinal herb Acorus gramineus by our lab, could alleviate neuronal overexcitation and improve the cognitive function of aged rats. In the present study, we investigated the effect and mechanism of α-asaronol on myelination in a rat model of PWMI induced by HI. Notably, α-asaronol promoted OPC differentiation and myelination in the corpus callosum of PWMI rats. Meanwhile, the concentration of glutamate was significantly decreased, and the levels of PPARγ and glutamate transporter 1 (GLT-1) were increased by α-asaronol treatment. In vitro, it was also confirmed that α-asaronol increased GLT-1 expression and recruitment of the PPARγ coactivator PCG-1a in astrocytes under oxygen and glucose deprivation (OGD) conditions. The PPARγ inhibitor GW9662 significantly reversed the effect of α-asaronol on GLT-1 expression and PCG-1a recruitment. Interestingly, the conditioned medium from α-asaronol-treated astrocytes decreased the number of OPCs and increased the number of mature oligodendrocytes. These results suggest that α-asaronol can promote OPC differentiation and relieve dysmyelination by regulating glutamate levels via astrocyte PPARγ-GLT-1 signaling. Although whether α-asaronol binds to PPARγ directly or indirectly is not investigated here, this study still indicates that α-asaronol may be a promising small molecular drug for the treatment of myelin-related diseases.
Keywords: PPARγ; glutamic acid; oligodendrocyte precursor cells; white matter injury; α-asaronol.
Copyright © 2022 Ge, Zhen, Liu, Feng, Wang, Zhang, Wang, Sun, Zheng, Bai and Yao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
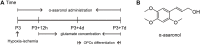




Similar articles
-
Alpha-asaronol promoted oligodendrocyte precursor cell differentiation and improved myelination as an activator PPARγ.Biomed Pharmacother. 2023 Jul;163:114815. doi: 10.1016/j.biopha.2023.114815. Epub 2023 May 3. Biomed Pharmacother. 2023. PMID: 37146420
-
A novel RIP1/RIP3 dual inhibitor promoted OPC survival and myelination in a rat neonatal white matter injury model with hOPC graft.Stem Cell Res Ther. 2021 Aug 18;12(1):462. doi: 10.1186/s13287-021-02532-1. Stem Cell Res Ther. 2021. PMID: 34407865 Free PMC article.
-
Revealing the Antiepileptic Effect of α-Asaronol on Pentylenetetrazole-Induced Seizure Rats Using NMR-Based Metabolomics.ACS Omega. 2022 Feb 9;7(7):6322-6334. doi: 10.1021/acsomega.1c06922. eCollection 2022 Feb 22. ACS Omega. 2022. PMID: 35224394 Free PMC article.
-
T0901317, a liver X receptor agonist, ameliorates perinatal white matter injury induced by ischemia and hypoxia in neonatal rats.Neurosci Lett. 2023 Jan 10;793:136994. doi: 10.1016/j.neulet.2022.136994. Epub 2022 Nov 29. Neurosci Lett. 2023. PMID: 36460235
-
Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms.Ment Retard Dev Disabil Res Rev. 2006;12(2):129-40. doi: 10.1002/mrdd.20107. Ment Retard Dev Disabil Res Rev. 2006. PMID: 16807910 Review.
Cited by
-
FGF21 Alleviates Hypoxic-Ischemic White Matter Injury in Neonatal Mice by Mediating Inflammation and Oxidative Stress Through PPAR-γ Signaling Pathway.Mol Neurobiol. 2025 Apr;62(4):4743-4768. doi: 10.1007/s12035-024-04549-y. Epub 2024 Nov 1. Mol Neurobiol. 2025. PMID: 39485628
-
Safety assessment of Acori Tatarinowii Rhizoma: acute and subacute oral toxicity.Front Pharmacol. 2024 Mar 19;15:1377876. doi: 10.3389/fphar.2024.1377876. eCollection 2024. Front Pharmacol. 2024. PMID: 38567357 Free PMC article.
-
Cardioprotective Effects of α-Asarone Against Hexavalent Chromium-Induced Oxidative Damage in Mice.Drug Des Devel Ther. 2024 Jul 30;18:3383-3397. doi: 10.2147/DDDT.S464334. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39100222 Free PMC article.
-
Neuroprotective Strategies for Stroke by Natural Products: Advances and Perspectives.Curr Neuropharmacol. 2023;21(11):2283-2309. doi: 10.2174/1570159X21666230717144752. Curr Neuropharmacol. 2023. PMID: 37458258 Free PMC article. Review.
-
Understanding Acquired Brain Injury: A Review.Biomedicines. 2022 Sep 2;10(9):2167. doi: 10.3390/biomedicines10092167. Biomedicines. 2022. PMID: 36140268 Free PMC article. Review.
References
-
- Back S. A., Craig A., Kayton R. J., Luo N. L., Meshul C. K., Allcock N., et al. (2007). Hypoxia-ischemia Preferentially Triggers Glutamate Depletion from Oligodendroglia and Axons in Perinatal Cerebral white Matter. J. Cereb. Blood Flow Metab. 27 (2), 334–347. 10.1038/sj.jcbfm.9600344 - DOI - PubMed
-
- Back S. A., Luo N. L., Borenstein N. S., Levine J. M., Volpe J. J., Kinney H. C. (2001). Late Oligodendrocyte Progenitors Coincide with the Developmental Window of Vulnerability for Human Perinatal white Matter Injury. J. Neurosci. 21 (4), 1302–1312. 10.1523/jneurosci.21-04-01302.2001 - DOI - PMC - PubMed
-
- Bai Y., He X., Bai Y., Sun Y., Zhao Z., Chen X., et al. (2019). Polygala Tenuifolia-Acori Tatarinowii Herbal Pair as an Inspiration for Substituted Cinnamic α-asaronol Esters: Design, Synthesis, Anticonvulsant Activity, and Inhibition of Lactate Dehydrogenase Study. Eur. J. Med. Chem. 183, 111650. 10.1016/j.ejmech.2019.111650 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

