Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS
- PMID: 35401230
- PMCID: PMC8990839
- DOI: 10.3389/fphar.2022.851246
Adverse Event Profiles of PARP Inhibitors: Analysis of Spontaneous Reports Submitted to FAERS
Abstract
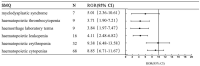
Background: Several poly ADP ribose polymerase inhibitors (PARPis) are currently approved for the treatment of a variety of cancers. The safety profile of PARPis has not yet been systemically analyzed in the real world. We conducted this pharmacovigilance analysis using the US FDA's Adverse Event Reporting System (FAERS) database to explore the difference in adverse events (AEs) among PARPis. Methods: FAERS data (December 2014 to October 2021) were searched for reports of all FDA-approved PARPis across all indications. We used the standardized MedDRA query (SMQ) generalized search AEs on the preferred term (PT) level based on case reports. After filtering duplicate reports, disproportionality analysis was used to detect safety signals by calculating reporting odds ratios (ROR). Reports were considered statistically significant if the 95% confidence interval did not contain the null value. Results: Within the standardized MedDRA queries, significant safety signals were found, including those for olaparib [blood premalignant disorders (ROR = 17.06)], rucaparib [taste and smell disorders (ROR = 9.17)], niraparib [hematopoietic throbocytopenia (ROR = 28.2)], and talazoparib [hematopoietic erythropenia (ROR = 9.38)]. For AEs on the PT level, we found several significant signals, including platelet count decreased with niraparib (ROR = 52.78); red blood cell count decreased with niraparib (ROR = 70.47) and rucaparib (ROR = 15.09); myelodysplastic syndrome with olaparib (ROR = 35.47); acute myeloid leukaemia with olaparib (ROR = 25.14); blood pressure fluctuation with niraparib (ROR = 20.54); lymphangioleiomyomatosis with niraparib (ROR = 471.20); photosensitivity reaction with niraparib (ROR = 21.77) and rucaparib (ROR = 18.92); renal impairment with rucaparib (ROR = 33.32); and interstitial lung disease with Olaparib (ROR = 11.31). All the detected safety signals were confirmed using signals of disproportionality reporting methods. Conclusion: PARPis differed in their safety profile reports. The analysis of the FAERS database revealed significant safety signals that matched previously published case reports, including serious gastrointestinal, blood and lymphatic system, cardiovascular and respiratory complications, which require individualized drug administration according to patients' conditions.
Keywords: FDA adverse events reporting system; PARP inhibitors; adverse events; reporting odds ratios; signal detection.
Copyright © 2022 Tian, Chen, Gai, He, Jiang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Comment in
-
Commentary: Adverse event profiles of PARP inhibitors: analysis of spontaneous reports submitted to FAERS.Front Pharmacol. 2023 Aug 16;14:1241524. doi: 10.3389/fphar.2023.1241524. eCollection 2023. Front Pharmacol. 2023. PMID: 37663271 Free PMC article. No abstract available.
Similar articles
-
Postmarketing safety of anaplastic lymphoma kinase (ALK) inhibitors: an analysis of the FDA Adverse Event Reporting System (FAERS).ESMO Open. 2021 Dec;6(6):100315. doi: 10.1016/j.esmoop.2021.100315. Epub 2021 Dec 2. ESMO Open. 2021. PMID: 34864500 Free PMC article.
-
Cardiovascular adverse events associated with PARP inhibitors for ovarian cancer: a real world study (RWS) with Bayesian disproportional analysis based on the FDA adverse event reporting system (FAERS).Expert Opin Drug Saf. 2025 Sep;24(9):1039-1046. doi: 10.1080/14740338.2024.2390640. Epub 2024 Aug 12. Expert Opin Drug Saf. 2025. PMID: 39132853
-
PARP inhibitor-related acute renal failure: a real-world study based on the FDA adverse event reporting system database.Expert Opin Drug Saf. 2024 Nov;23(11):1463-1471. doi: 10.1080/14740338.2024.2376690. Epub 2024 Jul 8. Expert Opin Drug Saf. 2024. PMID: 38967020
-
Comparative Efficacy and Safety of Poly (ADP-Ribose) Polymerase Inhibitors in Patients With Ovarian Cancer: A Systematic Review and Network Meta-Analysis.Front Oncol. 2022 Jun 8;12:815265. doi: 10.3389/fonc.2022.815265. eCollection 2022. Front Oncol. 2022. PMID: 35756600 Free PMC article.
-
Analysis of Spontaneous Postmarket Case Reports Submitted to the FDA Regarding Thromboembolic Adverse Events and JAK Inhibitors.Drug Saf. 2018 Apr;41(4):357-361. doi: 10.1007/s40264-017-0622-2. Drug Saf. 2018. PMID: 29196988 Review.
Cited by
-
The role of Poly-ADP ribose polymerase (PARP) enzymes in chemotherapy-induced cognitive impairments - parallels with other neurodegenerative disorders.Front Pharmacol. 2025 Jun 9;16:1615843. doi: 10.3389/fphar.2025.1615843. eCollection 2025. Front Pharmacol. 2025. PMID: 40552150 Free PMC article. Review.
-
Prostate Cancer Treatments and Their Effects on Male Fertility: Mechanisms and Mitigation Strategies.J Pers Med. 2025 Aug 7;15(8):360. doi: 10.3390/jpm15080360. J Pers Med. 2025. PMID: 40863422 Free PMC article. Review.
-
Current Therapeutic Strategies for Metastatic Triple-Negative Breast Cancer: From Pharmacists' Perspective.J Clin Med. 2022 Oct 12;11(20):6021. doi: 10.3390/jcm11206021. J Clin Med. 2022. PMID: 36294342 Free PMC article. Review.
-
Adverse events associated with oseltamivir and baloxavir marboxil in against influenza virus therapy: A pharmacovigilance study using the FAERS database.PLoS One. 2024 Nov 13;19(11):e0308998. doi: 10.1371/journal.pone.0308998. eCollection 2024. PLoS One. 2024. PMID: 39536015 Free PMC article.
-
BRCA and Beyond: Impact on Therapeutic Choices Across Cancer.Cancers (Basel). 2024 Dec 24;17(1):8. doi: 10.3390/cancers17010008. Cancers (Basel). 2024. PMID: 39796639 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

