Potential Mechanisms for Why Not All Antipsychotics Are Able to Occupy Dopamine D3 Receptors in the Brain in vivo
- PMID: 35401257
- PMCID: PMC8987915
- DOI: 10.3389/fpsyt.2022.785592
Potential Mechanisms for Why Not All Antipsychotics Are Able to Occupy Dopamine D3 Receptors in the Brain in vivo
Abstract
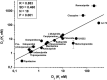
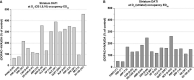
Dysfunctions of the dopaminergic system are believed to play a major role in the core symptoms of schizophrenia such as positive, negative, and cognitive symptoms. The first line of treatment of schizophrenia are antipsychotics, a class of medications that targets several neurotransmitter receptors in the brain, including dopaminergic, serotonergic, adrenergic and/or muscarinic receptors, depending on the given agent. Although the currently used antipsychotics display in vitro activity at several receptors, majority of them share the common property of having high/moderate in vitro affinity for dopamine D2 receptors (D2Rs) and D3 receptors (D3Rs). In terms of mode of action, these antipsychotics are either antagonist or partial agonist at the above-mentioned receptors. Although D2Rs and D3Rs possess high degree of homology in their molecular structure, have common signaling pathways and similar in vitro pharmacology, they have different in vivo pharmacology and therefore behavioral roles. The aim of this review, with summarizing preclinical and clinical evidence is to demonstrate that while currently used antipsychotics display substantial in vitro affinity for both D3Rs and D2Rs, only very few can significantly occupy D3Rs in vivo. The relative importance of the level of endogenous extracellular dopamine in the brain and the degree of in vitro D3Rs receptor affinity and selectivity as determinant factors for in vivo D3Rs occupancy by antipsychotics, are also discussed.
Keywords: D2 receptor; D3 receptor; antipsychotics; brain occupancy; dopamine; schizophrenia.
Copyright © 2022 Kiss, Krámos and Laszlovszky.
Conflict of interest statement
BKi, BKr, and IL were employees of Gedeon Richter Plc.
Figures


Similar articles
-
Selective overexpression of dopamine D3 receptors in the striatum disrupts motivation but not cognition.Biol Psychiatry. 2014 Nov 15;76(10):823-31. doi: 10.1016/j.biopsych.2013.11.023. Epub 2013 Dec 5. Biol Psychiatry. 2014. PMID: 24387821 Free PMC article.
-
Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding.Psychopharmacology (Berl). 1996 Mar;124(1-2):57-73. doi: 10.1007/BF02245606. Psychopharmacology (Berl). 1996. PMID: 8935801
-
Neuronal Dopamine D3 Receptors: Translational Implications for Preclinical Research and CNS Disorders.Biomolecules. 2021 Jan 14;11(1):104. doi: 10.3390/biom11010104. Biomolecules. 2021. PMID: 33466844 Free PMC article. Review.
-
The role of dopamine D3 receptors in the mechanism of action of cariprazine.CNS Spectr. 2020 Jun;25(3):343-351. doi: 10.1017/S109285291900083X. Epub 2019 Apr 23. CNS Spectr. 2020. PMID: 31010452 Review.
-
Bitropic D3 Dopamine Receptor Selective Compounds as Potential Antipsychotics.Curr Pharm Des. 2015;21(26):3700-24. doi: 10.2174/1381612821666150724100830. Curr Pharm Des. 2015. PMID: 26205291 Review.
Cited by
-
Antipsychotic-induced bone loss: the role of dopamine, serotonin and adrenergic receptor signalling.Front Cell Dev Biol. 2023 May 25;11:1184550. doi: 10.3389/fcell.2023.1184550. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37305679 Free PMC article. Review.
-
D3 Receptor-Targeted Cariprazine: Insights from Lab to Bedside.Int J Mol Sci. 2024 May 23;25(11):5682. doi: 10.3390/ijms25115682. Int J Mol Sci. 2024. PMID: 38891871 Free PMC article. Review.
-
Substance Addiction Rehabilitation Drugs.Pharmaceuticals (Basel). 2024 May 10;17(5):615. doi: 10.3390/ph17050615. Pharmaceuticals (Basel). 2024. PMID: 38794185 Free PMC article. Review.
-
Third-Generation Antipsychotics and Lurasidone in the Treatment of Substance-Induced Psychoses: A Narrative Review.Healthcare (Basel). 2024 Jan 29;12(3):339. doi: 10.3390/healthcare12030339. Healthcare (Basel). 2024. PMID: 38338224 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

