Deciphering Genetic Susceptibility to Tuberculous Meningitis
- PMID: 35401413
- PMCID: PMC8993185
- DOI: 10.3389/fneur.2022.820168
Deciphering Genetic Susceptibility to Tuberculous Meningitis
Abstract
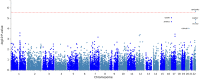
Tuberculous meningitis (TBM) is the most severe form of extrapulmonary tuberculosis (TB) that arises when a caseating meningeal granuloma discharges its contents into the subarachnoid space. It accounts for ~1% of all disease caused by Mycobacterium tuberculosis and the age of peak incidence is from 2-4 years. The exact pathogenesis of TBM is still not fully understood and the mechanism(s) by which the bacilli initially invade the blood-brain-barrier are still to be elucidated. This study investigated the involvement of the host genome in TBM susceptibility, by considering common variants (minor allele frequency (MAF) >5%) using microarray genotyping and rare variants (MAF <1%) via exome sequencing. A total of 123 TBM cases, 400 pulmonary TB (pTB) cases and 477 healthy controls were genotyped on the MEGA array. A genome-wide association study (GWAS) comparing 114 TBM cases to 395 healthy controls showed no association with TBM susceptibility. A second analysis comparing 114 TBM cases to 382 pTB cases was conducted to investigate variants associated with different TB phenotypes. No significant associations were found with progression from pTB to TBM. Ten TBM cases and 10 healthy controls were exome sequenced. Gene set association tests SKAT-O and SKAT Common Rare were used to assess the association of rare SNPs and the cumulative effect of both common and rare SNPs with susceptibility to TBM, respectively. Ingenuity Pathway Analysis (IPA) of the top-hits of the SKAT-O analysis showed that NOD2 and CYP4F2 are both important in TBM pathogenesis and highlighted these as targets for future study. For the SKAT Common Rare analysis Centriolar Coiled-Coil Protein 110 (CCP110) was nominally associated (p = 5.89x10-6) with TBM susceptibility. In addition, several top-hit genes ascribed to the development of the central nervous system (CNS) and innate immune system regulation were identified. Exome sequencing and GWAS of our TBM cohort has identified a single previously undescribed association of CCP110 with TBM susceptibility. These results advance our understanding of TBM in terms of both variants and genes that influence susceptibility. In addition, several candidate genes involved in innate immunity have been identified for further genotypic and functional investigation.
Keywords: exome sequencing; genome-wide association study; microarray; pulmonary tuberculosis; tuberculous meningitis.
Copyright © 2022 Schurz, Glanzmann, Bowker, van Toorn, Solomons, Schoeman, van Helden, Kinnear, Hoal and Möller.
Conflict of interest statement
NB is currently an employee and shareholder of GlaxoSmithKline (Stevenage, UK). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

